
Interstitial Cystitis Hub
The information, resources, and external links provided in this Interstitial Cystitis (IC) Reference Section are offered solely for educational and informational purposes. While we strive to ensure that all referenced materials are accurate, credible, and drawn from reputable medical and scientific sources, we do not endorse, guarantee, or assume responsibility for the content, accuracy, completeness, or reliability of any third-party website or publication. External links are provided as a convenience to our visitors and do not imply affiliation, sponsorship, or approval by our organization.
This website does not provide medical advice, diagnosis, or treatment. The information contained herein should not be considered a substitute for professional medical judgment. Always seek the advice of a qualified healthcare provider regarding any questions or concerns about your health, symptoms, or treatment options. Never disregard or delay seeking professional medical care based on information obtained from this site or any linked resources.
Use of any external site or referenced material is at your own risk. Our organization shall not be held liable for any loss, damage, injury, or adverse outcome arising from reliance on information or resources linked from this page. Content and links may change without notice, and it is the user’s responsibility to verify all information with a qualified professional.
Disclaimer for IC References
& External Links

Section Guide


Executive Summary
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic condition characterized by bladder- or pelvic-area pain associated with urinary urgency and frequency, in the absence of infection or other identifiable causes. Diagnosis is clinical and by exclusion, and management requires individualized, multimodal care guided by shared decision-making. This report distills current definitions, epidemiology, symptom patterns, diagnostic work-up, guideline-recommended therapies, self-management strategies, support resources, and late-breaking research
Definitions & Epidemiology
Definition: The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the American Urological Association (AUA) describe IC/BPS as chronic bladder-related pain with lower urinary tract symptoms for ≥6 weeks (AUA) to ≥6 months (ESSIC), without infection or other clear causes.
Phenotypes: IC with Hunner lesions (distinct inflammatory lesion seen on cystoscopy) vs. non-Hunner IC/BPS — these behave differently and may respond to different therapies.
Prevalence: U.S. prevalence ≈0.87% overall (~1.08% women; ~0.66% men).
Symptoms and Diagnosis
Core Symptom Profile
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is defined clinically by a constellation of bladder-related symptoms, most prominently:
Pain, pressure, or discomfort localized to the bladder, pelvis, or urethra, often increasing with bladder filling and partially relieved after voiding.
Urinary urgency — a compelling need to urinate due to pain/pressure, not just fear of leakage.
Increased urinary frequency — daytime and nighttime urination often exceeding normal limits (many patients void >8 times/day).
Nocturia — waking at night to urinate.
Dyspareunia — pain during or after sexual intercourse, more common in women but may affect men as pelvic pain after ejaculation.
Pelvic floor tenderness — muscle spasm or trigger points in pelvic floor muscles upon examination.
Flares and remissions — symptom intensity may wax and wane, often triggered by diet, stress, hormonal changes, or physical activity.
Women ████████████████████ 1.08%
Men ████████████████ 0.66%
Source: Anger et al., 2022
Overall U.S. prevalence ≈ 0.87%.
Differentiating Pain from Overactive Bladder (OAB)
Unlike OAB, urgency in IC/BPS is typically driven by pain or discomfort rather than fear of leakage, and urgency is not always relieved by voiding.
Common Symptom Patterns Reported by Patients
Pain Triggered by:
- Bladder filling
- Stress
- Menstrual cycle
- Certain foods/drinks
Relieved By:
- Voiding
- Heat application
- Pelvic rest
Diagnostic Approach
IC/BPS is a diagnosis of exclusion, meaning other causes of the symptoms must be ruled out before confirming the condition.
1. Clinical History
Symptom onset, duration (≥6 weeks per AUA; ≥6 months per ESSIC)
Pain location, severity, relation to bladder filling/emptying
Urinary frequency/urgency/nocturia patterns
Flare triggers and alleviating factors
Associated comorbidities (IBS, fibromyalgia, chronic fatigue syndrome)
2. Physical Examination
Abdominal and suprapubic palpation
Pelvic exam in women to assess pelvic floor tension, tenderness, and other gynecologic causes
Digital rectal exam in men to rule out prostate tenderness or enlargement
3. Laboratory Testing
Urinalysis and urine culture — exclude urinary tract infection
Urine cytology if hematuria or malignancy risk factors present
4. Post-Void Residual (PVR)
Simple ultrasound or catheter measurement to detect incomplete bladder emptying
5. Cystoscopy
Recommended if hematuria, risk factors for bladder cancer, or to evaluate for Hunner lesions
Hunner lesions appear as inflamed, bleeding patches on bladder mucosa and are pathognomonic for “Hunner lesion IC” phenotype
6. Optional/Research Tests
Potassium sensitivity test (no longer routinely recommended)
Urodynamics (if diagnostic uncertainty with OAB)
Investigational biomarkers: antiproliferative factor (APF), altered HB-EGF/EGF ratios
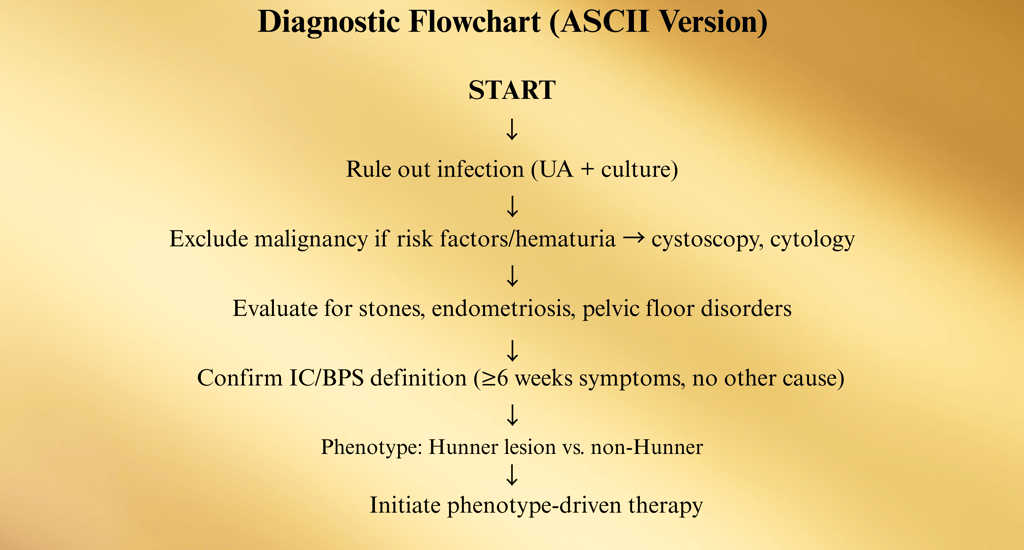
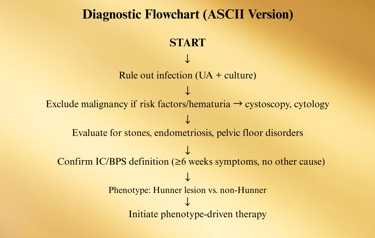
Diagnostic Approach
Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) is a diagnosis of exclusion, meaning it is confirmed only after other potential causes of bladder pain and urinary symptoms have been ruled out. The diagnostic pathway blends patient history, physical examination, laboratory workup, and selective imaging or cystoscopy.
1. Comprehensive Clinical History
Symptom Duration:
AUA Criteria: Persistent bladder pain/pressure with urinary symptoms for ≥6 weeks.
ESSIC Criteria: Symptoms for ≥6 months.
Pain Characteristics:
Location (suprapubic, pelvic, urethral, perineal, low back)
Relation to bladder filling and emptying
Aggravating factors (dietary triggers, stress, hormonal changes, exercise)
Relieving factors (voiding, heat, pelvic rest)
Urinary Symptoms:
Daytime frequency (often >8 times/day)
Nocturia
Urgency driven by discomfort (not fear of leakage)
Associated Symptoms:
Dyspareunia
Pelvic floor muscle spasm or tenderness
Fatigue, muscle aches, gastrointestinal complaints
Past Medical History:
Comorbid pain syndromes (fibromyalgia, IBS, chronic fatigue syndrome)
Gynecologic or urologic procedures
History of pelvic radiation or chemotherapy
2. Physical Examination
Abdominal Exam:
Suprapubic tenderness
Palpable bladder distension (if present)
Pelvic Exam (Females):
Evaluate for pelvic floor hypertonicity, tenderness, trigger points
Rule out endometriosis, pelvic masses, vulvodynia
Rectal Exam (Males):
Assess prostate size and tenderness
Palpate pelvic floor muscles for spasm
Neurological Exam:
Lower limb reflexes and sensation (if neurogenic cause suspected)
3. Laboratory Testing
Urinalysis: Look for pyuria, hematuria
Urine Culture: Rule out urinary tract infection
Urine Cytology: Recommended in patients >40 years or with risk factors for bladder cancer
Sexually Transmitted Infection (STI) Testing: If urethritis is suspected
4. Post-Void Residual (PVR) Measurement
Performed via bladder ultrasound or catheterization
High residual volumes suggest bladder outlet obstruction, neurogenic bladder, or severe detrusor underactivity — not typical for IC/BPS
5. Cystoscopy
Indications:
Hematuria
Suspicion of bladder cancer
High-risk patients (age >40, smokers, occupational exposures)
Severe or refractory symptoms
Findings:
Hunner lesions (inflamed, bleeding areas on bladder mucosa) → diagnostic for “Hunner lesion IC” phenotype
Glomerulations (pinpoint hemorrhages) are nonspecific but may be present after hydrodistention
Therapeutic Role:
Lesion-directed treatment (fulguration, steroid injection) may be performed during cystoscopy
6. Optional & Research-Based Tests
Potassium Sensitivity Test (PST): Formerly used, now largely abandoned due to discomfort and poor specificity.
Urodynamics: Reserved for atypical cases or when OAB is a strong alternative diagnosis.
Biomarkers (Research Use):
Antiproliferative Factor (APF) — inhibits urothelial cell growth
Altered Heparin-Binding Epidermal Growth Factor (HB-EGF) levels
These remain investigational and are not part of standard clinical care
Treatment and Care
Principles of Management
Individualization: No single treatment is effective for all patients.
Escalation/De-escalation: Move up or down therapy levels depending on response and tolerance.
Multimodal Therapy: Combining behavioral, pharmacologic, and procedural interventions is often more effective than monotherapy.
Patient Education: Explaining the nature of IC/BPS, possible triggers, and treatment expectations is essential to adherence.
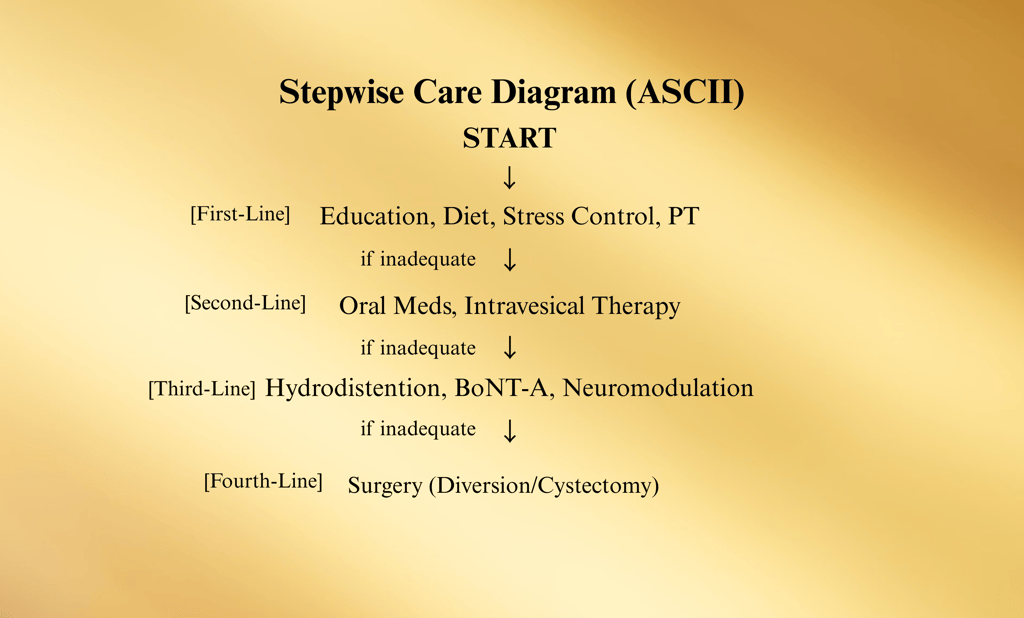
Stepwise Treatment Pathway (ASCII)
Step 1: Education, Lifestyle & Behavioral Modification
Step 2: Oral Medications and/or Intravesical Instillations
Step 3: Minimally Invasive Procedures
Step 4: Major Surgery (only for end-stage cases)
(Arrows between steps indicate escalation or de-escalation as needed.)
Management of Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) is individualized and multimodal, with therapy chosen based on patient phenotype, symptom severity, comorbidities, and patient preference. The American Urological Association (AUA) recommends a stepwise approach with shared decision-making at every stage.
Step 1 – First-Line Conservative Measures
Goal: Symptom reduction through lifestyle and behavioral interventions.
Patient Education
Explain chronic nature of IC/BPS and realistic expectations.
Review phenotypes (Hunner vs. non-Hunner) and implications for treatment.
Dietary Modification
Avoid common bladder irritants: acidic fruits, tomatoes, spicy foods, alcohol, caffeine, carbonated drinks, artificial sweeteners.
Implement an IC elimination diet for 4–6 weeks, then reintroduce foods gradually to identify triggers.
Refer to ICN 2025 Food List for safe/trigger food guidance.
Bladder Training & Timed Voiding
Gradually increase time between voids to reduce frequency and retrain bladder signaling.
Stress Management & Relaxation Techniques
Mindfulness, yoga, biofeedback, or cognitive behavioral therapy (CBT).
Pelvic Floor Physical Therapy
Focus on myofascial release and relaxation rather than strengthening (Kegels are generally avoided unless indicated for another condition).
Step 2 – Second-Line Medical Therapies
Goal: Pharmacologic symptom control when conservative measures are insufficient.
Oral Medications (AUA-listed options):
Amitriptyline (10–75 mg at night): Neuromodulator and antidepressant with analgesic properties.
Cimetidine: H2 receptor antagonist; may reduce bladder pain and frequency.
Hydroxyzine: Antihistamine; targets mast cell activity in bladder wall.
Pentosan Polysulfate Sodium (PPS): Only FDA-approved oral agent; restores bladder glycosaminoglycan layer.
Note: Risk of pigmentary maculopathy with long-term use — baseline and annual ophthalmology exams recommended.
Intravesical Instillations (delivered directly into bladder):
Alkalinized Lidocaine: Provides local anesthetic effect for flares.
Heparin: Mimics glycosaminoglycan layer; often combined with lidocaine.
Dimethyl Sulfoxide (DMSO): FDA-approved for IC; anti-inflammatory, analgesic, muscle-relaxant effects.
Step 3 – Minimally Invasive Procedures
Goal: Address refractory symptoms with targeted interventions.
Hydrodistention (Low Pressure, Short Duration)
Can provide temporary symptom relief and may help identify Hunner lesions.
Hunner Lesion Treatment
Fulguration (cauterization) or resection via cystoscopy, often with steroid injection.
Typically yields significant symptom relief in true Hunner lesion disease.
Intravesical Botulinum Toxin A (BoNT-A)
Injections into bladder wall at 100 U dose have RCT-level evidence of improving pain and urgency.
Risks: urinary retention (5–15% of patients), may require intermittent catheterization.
Neuromodulation
Sacral Neuromodulation (SNS): Implanted device modulates bladder nerve pathways.
Percutaneous Tibial Nerve Stimulation (PTNS): Outpatient minimally invasive alternative.
Transcutaneous Tibial Nerve Stimulation (TTNS): Non-invasive, home-based.
Step 4 – Major Surgery (Reserved for End-Stage, Refractory Cases)
Cystectomy with Urinary Diversion
Consider only after exhausting all other measures.
Rarely performed due to invasiveness and potential complications.
Adjunctive Therapies & Supportive Care
Pain Management: Multimodal analgesia including NSAIDs, gabapentinoids, or referral to pain specialists.
Complementary Therapies: Acupuncture, low-impact exercise (e.g., swimming, walking).
Psychological Support: Counseling to address depression, anxiety, and quality-of-life issues.

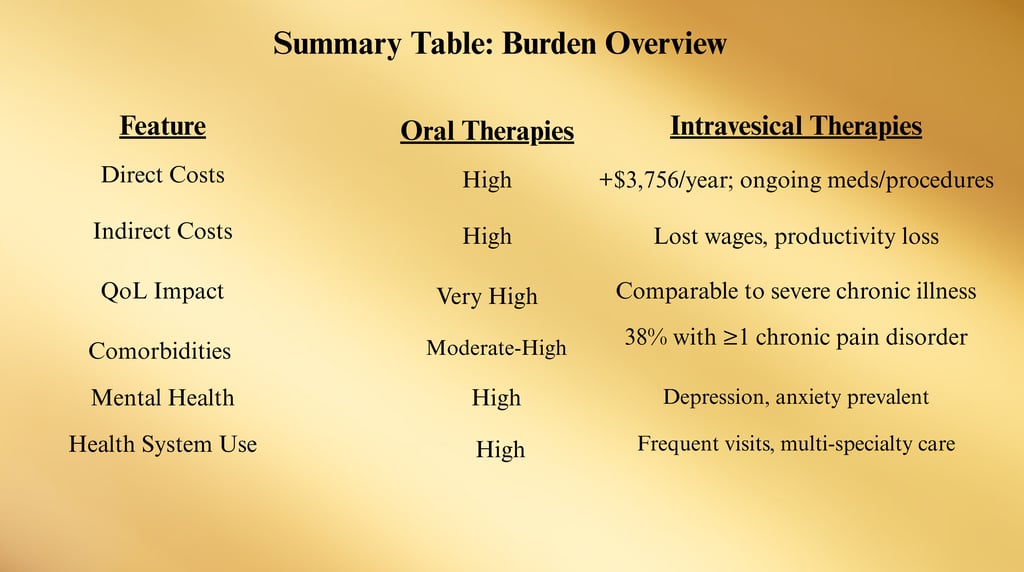
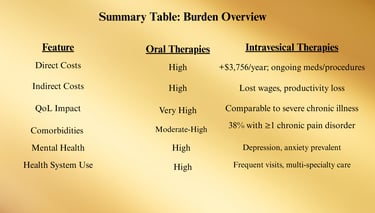
Burden: Costs and Comorbidities
Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) imposes a significant clinical and economic burden on patients, healthcare systems, and society. The impact extends beyond bladder-specific symptoms, affecting overall health, daily functioning, and quality of life.
1. Economic Burden
Direct Medical Costs
Patients with IC/BPS incur substantially higher healthcare expenses compared to matched controls due to:
Frequent outpatient visits (urology, gynecology, primary care, pain clinics)
Diagnostic testing (lab work, cystoscopy, imaging)
Medication costs (oral agents, intravesical agents, pain management drugs)
Procedural interventions (hydrodistention, BoNT-A, neuromodulation)
Chart: Average First-Year Direct Healthcare Costs (USD)
IC/BPS Patients ████████████████████████████████ $6,614
Matched Controls ████████████ $2,858
Source: Tung et al., 2017
Difference: ~$3,756 more in first-year costs for IC/BPS patients.
Indirect Costs
Lost wages due to absenteeism and reduced productivity (presenteeism).
Disability claims and early retirement in severe cases.
Increased mental health service utilization.
2. Quality-of-Life Impact
IC/BPS can cause a debilitating loss of quality of life comparable to or worse than that seen in end-stage renal disease and rheumatoid arthritis.
Sleep disturbance due to nocturia.
Limitation of social, sexual, and occupational activities.
Emotional distress — higher prevalence of depression and anxiety.
3. Comorbidities
Many patients with IC/BPS present with overlapping chronic pain and functional disorders, known as Urological Chronic Pelvic Pain Syndromes (UCPPS) overlap.
Chart: Comorbidity Rates in IC/BPS
≥1 Comorbidity ████████████████████ 38%
No Comorbidity ████████████████████████████████████ 62%
Source: Laden et al., 2021
Common Comorbid Conditions
Fibromyalgia — Widespread musculoskeletal pain, fatigue.
Irritable Bowel Syndrome (IBS) — Abdominal pain, altered bowel habits.
Chronic Fatigue Syndrome (CFS/ME) — Severe fatigue not improved by rest.
Migraine Headaches — Possibly linked via central sensitization mechanisms.
Vulvodynia — Chronic vulvar pain in women with IC/BPS.
4. Psychosocial Burden
Depression and Anxiety
Chronic pain and uncertainty about flares contribute to high psychiatric comorbidity rates.
Mental health symptoms can exacerbate perception of bladder pain.
Social Isolation
Unpredictable symptoms cause patients to avoid travel, public events, and sexual activity.
Fear of symptom flares often results in a narrowed lifestyle.
5. Impact on Health Systems
IC/BPS patients often become high utilizers of care due to:
Repeat visits for flares
Trial-and-error treatment courses
Need for multidisciplinary involvement (urology, pain, physical therapy, mental health)
Support and Advocacy
Living with Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) can be isolating and overwhelming. Support networks, advocacy organizations, and patient education resources are crucial for helping individuals manage symptoms, access care, and influence public policy.
1. Patient Education & Self-Advocacy
Empowering patients with accurate information improves adherence to treatment plans and reduces anxiety.
Track symptoms using bladder diaries or flare logs.
Prepare for appointments by listing questions and recent changes in symptoms.
Learn rights under the ADA for workplace accommodations if symptoms limit job performance.
2. National & International Advocacy Organizations
Interstitial Cystitis Association (ICA)
Website: https://www.ichelp.org/
Provides patient education, webinars, downloadable guides, and a provider registry.
Advocates for increased research funding through NIH and private sources.
Interstitial Cystitis Network (ICN)
Website: https://www.ic-network.com/
Offers the annually updated IC Food List, flare management tips, and moderated support forums.
Publishes newsletters with research updates and clinical trial opportunities.
Pain Alliance Europe
Website: https://www.painallianceeurope.eu/
Umbrella organization representing chronic pain patients, including those with IC/BPS, at the EU policy level.
International Pain Foundation (iPain)
Website: https://internationalpain.org/
Global education and advocacy for chronic pain conditions.
Organizes patient stories campaigns to raise awareness.
Rare Disease Day Network
Website: https://www.rarediseaseday.org/
Annual global event to raise awareness and improve research funding for rare and under-recognized conditions like IC/BPS.
3. Local and Community-Based Support
Hospital/Clinic Support Groups: Many urology departments host monthly IC support meetings.
Peer Mentorship Programs: Match newly diagnosed patients with experienced IC advocates for guidance.
Faith-Based and Community Centers: Some offer free support circles for chronic pain sufferers.
4. Online Support Communities
Social Media Groups
Facebook, Reddit, and Instagram have large IC communities sharing daily tips, diet modifications, and emotional support.
Tip: Encourage patients to verify medical advice from these groups with healthcare professionals.
Nonprofit Forums
ICN Forum — long-standing, moderated discussion board with searchable archives.
Inspire.com IC/BPS Community — integrated with several advocacy groups.
5. Advocacy in Healthcare Policy
Push for insurance coverage expansion to include pelvic floor physical therapy, mental health care, and integrative therapies.
Support federal legislation for increased chronic pain research funding.
Participate in NIH public comment periods to ensure IC/BPS research priorities reflect patient needs.


First-Line Foundations
First-line interventions for Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) are conservative, non-invasive measures aimed at reducing symptoms, improving bladder health, and preventing escalation to more invasive therapies.
These steps form the cornerstone of care for all patients — regardless of disease severity or phenotype — and are typically maintained even if higher-tier treatments are introduced later.
1. Patient Education
The first and most critical step is ensuring the patient understands:
IC/BPS is a chronic condition — treatments aim to reduce symptoms, not cure.
Flares are common, but often manageable with trigger control and early intervention.
Treatment is trial-and-error — what works for one patient may not work for another.
Symptom relief is usually gradual — many interventions take weeks to months to work.
Educational Tools:
AUA’s IC/BPS Patient Fact Sheet
ICA’s Living with IC Handbook
IC Network’s Food List & Flare Management Guide
2. Lifestyle & Behavioral Modification
Dietary Adjustments (IC Elimination Diet)
Avoid common bladder irritants: coffee, tea, soda, alcohol, citrus fruits, tomatoes, vinegar, hot spices, artificial sweeteners.
Trial elimination: Remove high-risk foods for 4–6 weeks, then reintroduce one at a time to identify triggers.
Safe food emphasis: pears, blueberries, plain rice, most leafy greens, plain chicken/turkey, pure water.
Bladder Training & Timed Voiding
Gradually increase intervals between urination by 15–30 minutes.
Helps reduce urgency and frequency over time by desensitizing bladder signaling pathways.
Stress Management
Stress is a major flare trigger due to effects on pelvic floor muscles and immune activity.
Recommended: mindfulness meditation, yoga, breathing exercises, guided imagery.
3. Physical Therapy (Pelvic Floor Focus)
Goal: Reduce pelvic floor hypertonicity and trigger point pain.
Myofascial release (internal and external)
Trigger point therapy for pelvic muscles
Breathing coordination to relax pelvic muscles during voiding
Biofeedback to improve muscle awareness
Note:
Kegel strengthening exercises are not recommended unless weakness is present without hypertonicity — most IC/BPS patients have tight pelvic floors, not weak ones.
4. Fluid Management
Avoid extremes:
Too little fluid → concentrated urine increases irritation.
Too much fluid → increases frequency and bladder workload.
Recommendation:
Moderate, steady hydration with filtered water throughout the day.
Avoid drinking large volumes close to bedtime to reduce nocturia.
5. Flare Management Strategies
Heat & Cold Therapy
Heating pads over the bladder/pelvic area for muscle relaxation.
Ice packs or cold compresses for numbing acute pain.
Over-the-Counter Options
Phenazopyridine (short-term use for acute pain) — note: can turn urine orange/red.
Prelief® (calcium glycerophosphate) to reduce acidity of foods/beverages.
First-Line Care Flowchart (ASCII)
Diagnosed IC/BPS
↓
Patient Education
↓
Lifestyle & Diet Adjustments
↓
Pelvic Floor Physical Therapy
↓
Bladder Training / Fluid Optimization
↓
Flare Management Plan
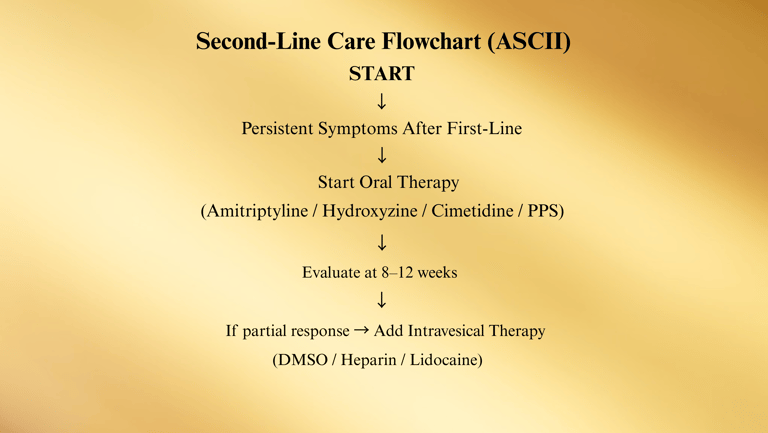
Second-Line Treatments
When first-line interventions (education, diet, stress control, pelvic floor therapy) are insufficient in managing symptoms, the American Urological Association (AUA) recommends progressing to second-line treatments.
These include oral medications and intravesical (bladder-administered) therapies — either individually or in combination.
1. Oral Pharmacologic Therapies
Amitriptyline (10–75 mg nightly)
Class: Tricyclic antidepressant (TCA)
Mechanism: Reduces nerve pain signaling, relaxes bladder, improves sleep.
Benefits: Pain reduction, improved nocturia, better sleep quality.
Side Effects: Dry mouth, constipation, drowsiness, weight gain.
Cimetidine (400 mg twice daily)
Class: H2 receptor antagonist
Mechanism: Decreases histamine activity in bladder tissue, reducing inflammation and urgency.
Benefits: Good safety profile, inexpensive.
Side Effects: Rare — dizziness, headache, diarrhea.
Hydroxyzine (25–50 mg nightly)
Class: Antihistamine
Mechanism: Reduces mast cell activity, which is believed to play a role in bladder inflammation.
Benefits: Especially effective in patients with allergies or seasonal flares.
Side Effects: Sedation, dry mouth, dizziness.
Pentosan Polysulfate Sodium (PPS) (100 mg 3x/day)
Class: Oral bladder protectant; only FDA-approved oral drug for IC/BPS.
Mechanism: Restores glycosaminoglycan (GAG) layer of bladder lining.
Benefits: Improves pain, urgency, and frequency in some patients after 3–6 months.
Risks: Long-term use linked to pigmentary maculopathy (eye damage) — baseline and annual eye exams recommended.
2. Intravesical Therapies (Bladder Instillations)
Dimethyl Sulfoxide (DMSO) (50% solution, instilled for 15–20 minutes)
Mechanism: Anti-inflammatory, analgesic, muscle relaxant.
FDA-Approved: Yes, for IC/BPS.
Notes: May cause temporary garlic-like odor on breath/skin.
Heparin (10,000–40,000 units in saline)
Mechanism: Mimics and restores damaged GAG layer.
Often Combined With: Lidocaine for pain relief.
Alkalinized Lidocaine (200–400 mg with sodium bicarbonate)
Mechanism: Local anesthetic — provides rapid pain relief during flares.
Use: Often as part of “rescue instillation” therapy.
Cocktail Instillations
Many centers combine heparin + lidocaine + sodium bicarbonate for a multi-action effect.
Instilled via catheter once or twice weekly, then tapered based on symptom control.
3. Combination Therapy
Rationale:
Combining oral and intravesical treatments can address symptoms through multiple mechanisms (nerve modulation, inflammation reduction, bladder lining repair).
Example Protocol:
Oral amitriptyline + PPS
Weekly intravesical heparin/lidocaine for 6 weeks
Gradual taper of instillations while maintaining oral therapy
4. Monitoring & Safety
Baseline Assessments: Eye exams for PPS; allergy history before hydroxyzine.
Follow-Up: Every 8–12 weeks to assess symptom response and adjust doses.
Flare Protocol: Patients should have a plan for acute symptom spikes — may include “rescue” instillation or short-term pain meds.

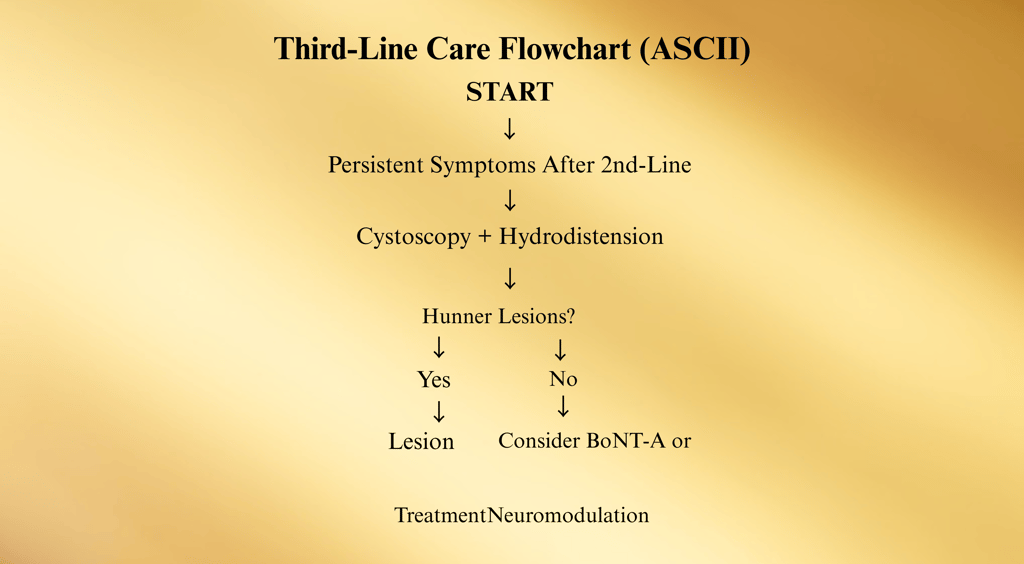
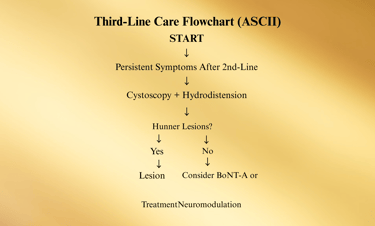
Third-Line Treatments
When IC/BPS symptoms persist despite first- and second-line therapies, the third-line step introduces minimally invasive procedural interventions.
These treatments aim to directly target bladder pathology, improve pain control, and enhance bladder capacity, while still avoiding irreversible surgical changes
1. Cystoscopy with Hydrodistention
Purpose:
Diagnostic: May reveal Hunner lesions or bladder mucosal abnormalities not visible otherwise.
Therapeutic: Low-pressure, short-duration hydrodistention can temporarily improve symptoms in some patients.
Technique:
Bladder is filled with sterile fluid under anesthesia at 60–80 cm H₂O pressure for less than 10 minutes.
Minimizes risk of overdistension damage.
Post-Procedure Effects:
Symptom relief in 30–50% of patients, often lasting 3–6 months.
Possible flare-up for 1–2 weeks after procedure.
2. Treatment of Hunner Lesions
Why It Matters:
Patients with Hunner lesions tend to respond poorly to non-targeted therapies but may have dramatic relief after lesion-directed treatment.
Treatment Options:
Fulguration: Electrocautery to destroy lesion tissue.
Laser Ablation: Nd:YAG or Holmium laser to precisely vaporize lesion.
Steroid Injection: Triamcinolone directly into lesion to reduce inflammation.
Evidence:
Many patients report pain reduction from 8–9/10 to 2–3/10 after lesion treatment, lasting months to years.
3. Intravesical Botulinum Toxin A (BoNT-A)
Mechanism:
Blocks acetylcholine release at neuromuscular junctions → relaxes bladder muscle and reduces pain signaling.
Protocol:
Typical dose: 100 units BoNT-A injected into 20 sites in the bladder wall under cystoscopic guidance.
Efficacy:
RCTs show significant improvement in urgency, frequency, and pain in IC/BPS patients.
Risks:
Temporary urinary retention (5–15% of patients) — may require self-catheterization for several weeks.
Possible urinary tract infection post-procedure.
4. Neuromodulation
Sacral Neuromodulation (SNS)
Mechanism: Implanted device delivers mild electrical impulses to sacral nerves (S3) to modulate bladder signaling pathways.
Process: Trial stimulation period followed by permanent implant if >50% symptom improvement.
Benefits: Reduces urgency, frequency, and pelvic pain.
Percutaneous Tibial Nerve Stimulation (PTNS)
Mechanism: Outpatient procedure stimulating tibial nerve (L4–S3 roots) via fine needle in ankle region.
Course: Weekly 30-minute sessions for 12 weeks, then maintenance every 3–4 weeks.
Advantages: Non-surgical, well tolerated, no implanted device.
Transcutaneous Tibial Nerve Stimulation (TTNS)
Similar to PTNS but uses surface electrodes — can be performed at home.
5. Patient Selection for Third-Line Therapies
Ideal candidates:
Persistent pain and urgency/frequency despite optimized oral + intravesical therapy.
No major medical contraindications to anesthesia (for BoNT-A or hydrodistention).
Motivated to follow post-procedure protocols.
Fourth-Line Treatments
Fourth-line therapies are major surgical interventions considered only when all conservative, pharmacologic, and minimally invasive measures have failed to provide adequate relief.
These are irreversible procedures that fundamentally alter bladder structure or function, and they carry substantial risks.
They are generally reserved for:
Severe, intractable pain
Markedly reduced bladder capacity (often <150 mL under anesthesia)
Confirmed bladder-centric disease without significant contribution from other pelvic pain syndromes
1. Augmentation Cystoplasty
Definition:
Surgical enlargement of the bladder by grafting a segment of intestine (ileum, sigmoid colon) into the bladder wall.
Purpose:
Increases bladder capacity and compliance, reducing urgency and frequency.
Considerations:
May be combined with removal of severely inflamed bladder dome tissue.
Some patients still require intermittent catheterization post-surgery.
Risks:
Mucus production from bowel segment (can lead to blockage or infection)
Electrolyte imbalances
Persistent or recurrent pain if symptoms are not purely bladder-based
2. Urinary Diversion with or without Cystectomy
A. Supravesical Diversion without Cystectomy
Urine is rerouted through an ileal conduit to a stoma (external urostomy bag).
Bladder is left in place — often done to avoid extensive pelvic dissection in high-risk patients.
Risk of persistent bladder pain if bladder remains in situ.
B. Urinary Diversion with Cystectomy
Bladder is surgically removed along with nearby pelvic tissue if necessary.
Urine is diverted either through an ileal conduit or a continent urinary reservoir (e.g., Indiana pouch).
Advantages:
Can provide complete pain relief in patients with purely bladder-origin symptoms.
Risks:
Major surgery with long recovery time
Possible complications: infection, hernia, stoma issues, bowel obstruction
Irreversible — lifelong change in urinary function
3. Orthotopic Neobladder Reconstruction
Definition:
Creation of a new bladder from intestinal tissue, connected to the urethra to allow voiding through the natural urinary channel.
Pros:
No external appliance needed.
Cons:
Requires intact urethral sphincter function.
Often associated with nighttime leakage; some patients require intermittent catheterization.
Potential for recurrence of pelvic pain despite bladder removal.
4. Candidate Evaluation Before Surgery
Confirm that symptoms are truly bladder-centric through urodynamics, hydrodistention, and exclusion of overlapping pain syndromes (pelvic floor dysfunction, vulvodynia, IBS).
Comprehensive psychological evaluation — surgery is not a guaranteed pain cure, and persistent pain is possible.
Extensive discussion with patient about irreversibility, lifestyle changes, and complication risks.
5. Post-Surgical Outcomes
Statistics:
Up to 80% of carefully selected patients report substantial pain relief after cystectomy.
20–30% may have persistent pain due to non-bladder pelvic pain syndromes.
Most require long-term follow-up with urology, stoma nurses, and sometimes pain specialists.
Maintenance & Monitoring


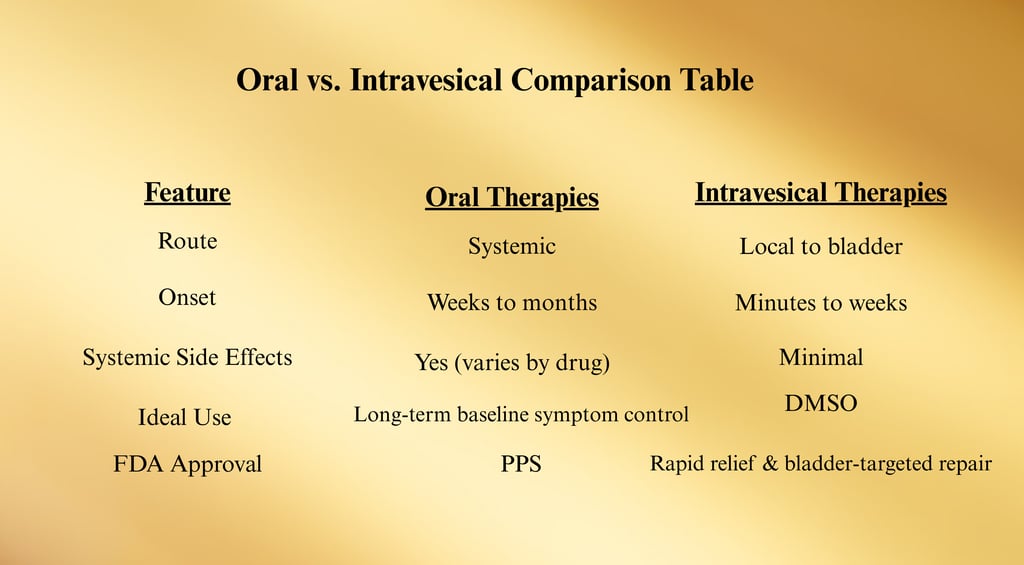
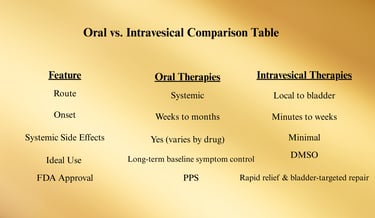
Oral and Intravesical Therapies
Oral and intravesical (bladder-administered) treatments form a major therapeutic tier for Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS), bridging the gap between conservative first-line care and procedural interventions.
They are often used together in combination protocols to target multiple mechanisms of the disease — bladder lining repair, nerve signal modulation, and inflammation control.
1. Oral Pharmacologic Therapies
Amitriptyline (10–75 mg nightly)
Class: Tricyclic antidepressant (TCA)
Mechanism: Modulates neuropathic pain, blocks histamine and muscarinic receptors, and improves sleep.
Efficacy: Multiple RCTs demonstrate reduction in urgency, pain, and nocturia within 4–6 weeks.
Adverse Effects: Dry mouth, constipation, weight gain, sedation; titrate slowly to minimize effects.
Hydroxyzine (25–50 mg nightly)
Class: H1 histamine receptor antagonist
Mechanism: Inhibits mast cell degranulation, reducing histamine-mediated bladder inflammation.
Indications: Particularly effective in patients with coexisting allergies or seasonal symptom flares.
Side Effects: Sedation, dizziness, dry mouth.
Cimetidine (400 mg twice daily)
Class: H2 receptor antagonist
Mechanism: Reduces histamine-driven inflammation in the bladder wall.
Evidence: Small studies show improvement in urgency and pain scores.
Side Effects: Headache, diarrhea, rare endocrine effects.
Pentosan Polysulfate Sodium (PPS) (100 mg three times daily)
Class: Oral bladder protectant — only FDA-approved oral drug for IC/BPS.
Mechanism: Replenishes the glycosaminoglycan (GAG) layer of bladder urothelium, protecting nerve endings from irritants.
Efficacy: Response rates of 28–32% in clinical trials; benefit may take 3–6 months to appear.
Risks: Pigmentary maculopathy after long-term use — baseline and annual retinal exams required.
2. Intravesical Therapies
Intravesical therapy delivers medication directly into the bladder via catheter, providing high local drug concentrations while limiting systemic exposure.
Dimethyl Sulfoxide (DMSO) (50% solution, instilled for 15–20 minutes)
Mechanism: Anti-inflammatory, analgesic, and smooth muscle relaxant.
Status: Only FDA-approved intravesical drug for IC/BPS.
Notes: Can cause temporary garlic-like odor for up to 48 hours.
Heparin (10,000–40,000 units in sterile saline)
Mechanism: Restores damaged GAG layer, reducing permeability and nerve exposure.
Use: Often part of “rescue instillation” cocktails.
Safety: Minimal systemic absorption; safe for long-term use.
Alkalinized Lidocaine (200–400 mg + sodium bicarbonate)
Mechanism: Numbs bladder wall nerve endings for rapid pain relief.
Role: Common for acute flares — relief within minutes to hours.
Cocktail Formulations
Example: Heparin + Lidocaine + Sodium Bicarbonate
Combines bladder lining repair with nerve blocking for both short-term and sustained symptom control.
3. Treatment Protocols
Induction Phase: 1–2 instillations per week for 6–8 weeks.
Maintenance Phase: Frequency reduced to once every 2–4 weeks, or as needed during flares.
Combination Strategy: Oral PPS or amitriptyline + intravesical therapy often yields better outcomes than monotherapy.
4. Monitoring & Safety
PPS: Eye exam before starting, repeat annually.
Amitriptyline: Monitor blood pressure, heart rhythm (baseline ECG for cardiac risk patients).
Intravesical Therapies: Monitor for urinary tract infection, catheter-related trauma.
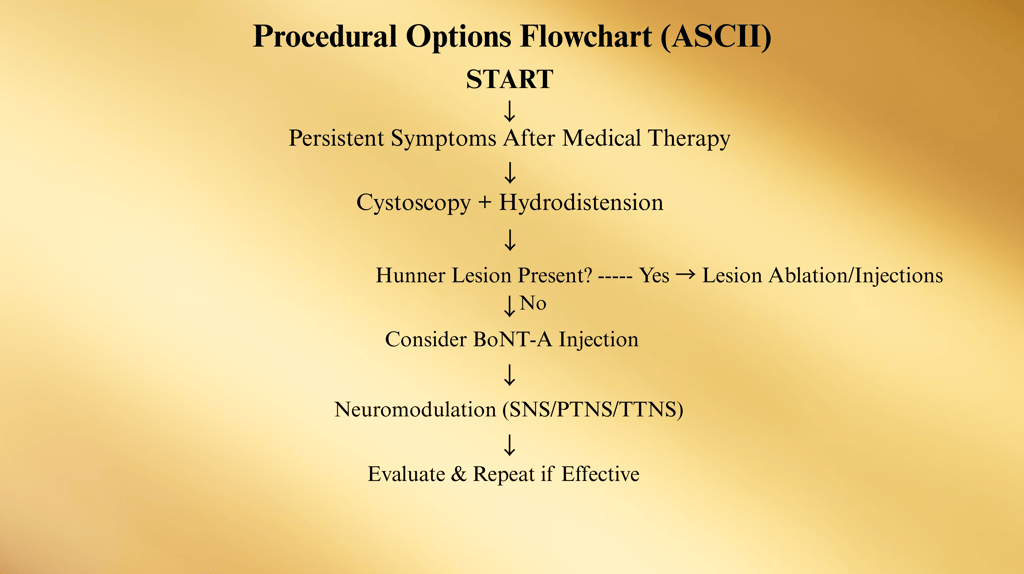
Procedural Options
Procedural treatments for Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) are considered when conservative measures, oral medications, and intravesical instillations do not achieve satisfactory symptom control.
They are typically third-line interventions per the American Urological Association (AUA) Guidelines and are designed to be less invasive than major surgery while offering direct, bladder-targeted relief.
1. Cystoscopy with Low-Pressure, Short-Duration Hydrodistention
Purpose
Diagnostic: Direct visualization of bladder mucosa to identify Hunner lesions, glomerulations, or abnormal vascular patterns.
Therapeutic: Gentle distention can improve capacity and temporarily reduce urgency/frequency.
Procedure
Performed under anesthesia.
Bladder filled with sterile fluid at 60–80 cm H₂O pressure for <10 minutes to avoid overdistension damage.
Evidence
Symptom relief reported in ~30–50% of patients, lasting 3–6 months.
Relief more likely in patients without significant pelvic floor dysfunction.
Risks
Temporary worsening of pain for several days post-procedure.
Rare bladder perforation if overdistended.
2. Hunner Lesion Treatment
Importance
Hunner lesions are a distinct IC/BPS subtype, highly responsive to lesion-directed therapy.
Options
Electrocautery (Fulguration): Burns away lesion tissue to promote healing.
Laser Ablation: Nd:YAG or Holmium laser used for precise removal.
Triamcinolone Injection: Direct steroid injection to reduce inflammation.
Outcomes
Significant pain reduction in many patients, often sustained for months to years.
Lesions may recur, requiring repeat treatment.
3. Intravesical Botulinum Toxin A (BoNT-A) Injections
Mechanism
Blocks acetylcholine release at nerve terminals, reducing detrusor overactivity and nociceptive signaling.
Protocol
100 units injected into 20 sites in the bladder wall under cystoscopic guidance.
Effect onset: 1–2 weeks; duration: 4–9 months.
Efficacy
RCTs demonstrate improvement in urgency, frequency, and pain scores.
Risks
Temporary urinary retention (5–15%) — patients may need to self-catheterize.
Possible urinary tract infection.
4. Neuromodulation
A. Sacral Neuromodulation (SNS)
Device: Implanted pulse generator sends mild electrical impulses to sacral nerve roots (S3).
Process: Trial stimulation period first; if >50% symptom improvement, permanent implant placed.
Benefits: Reduces urgency, frequency, and pelvic pain.
B. Percutaneous Tibial Nerve Stimulation (PTNS)
Method: Fine needle inserted near tibial nerve at ankle; connected to stimulator.
Course: 30-minute sessions weekly for 12 weeks, then monthly maintenance.
Advantages: Minimally invasive, no implanted device.
C. Transcutaneous Tibial Nerve Stimulation (TTNS)
Uses surface electrodes instead of needle; can be done at home.
5. Combination Procedural Therapy
Some patients benefit from combining hydrodistention with lesion ablation or following BoNT-A injections with neuromodulation.
Strategy individualized based on symptom profile, bladder findings, and patient preference.
6. Monitoring After Procedures
Follow-up at 4–6 weeks to assess symptom changes.
Bladder diaries can help track improvement.
Repeat procedures considered if benefits wane and patient tolerates them well.

Research and Future Developments
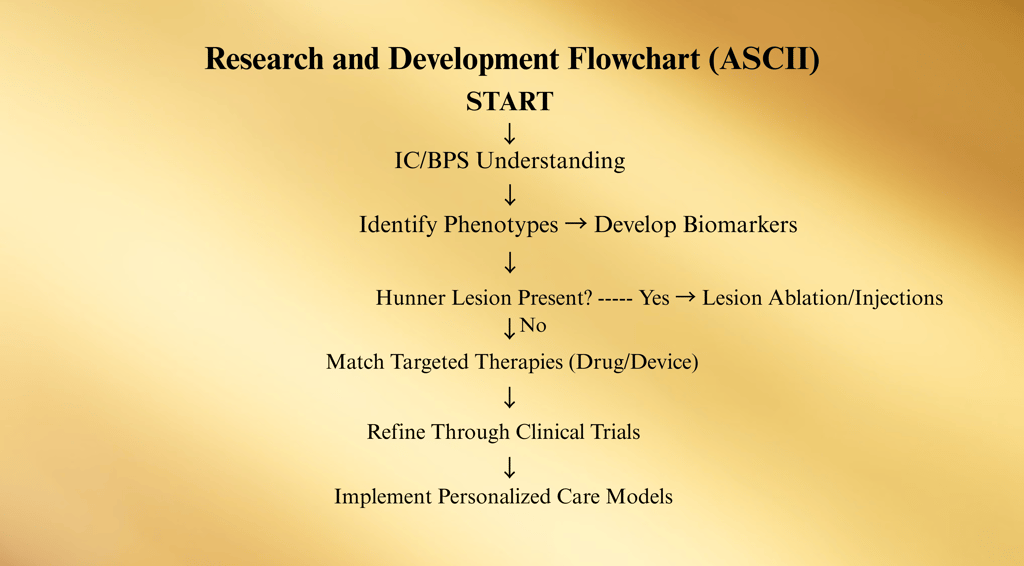
Research into Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) continues to evolve rapidly, focusing on better diagnostic accuracy, precision-targeted therapies, and understanding disease mechanisms at the cellular level.
Current efforts span multiple fields: basic science, drug development, device innovation, and patient-centered care models.
1. Pathophysiology Research
Recent studies have identified potential subtypes of IC/BPS, suggesting it is not a single disease but a spectrum disorder:
Bladder-centric phenotype — often with Hunner lesions, inflammatory markers, and epithelial defects.
Non–bladder-centric phenotype — overlaps with central sensitization syndromes (e.g., fibromyalgia, IBS, vulvodynia).
Ongoing Investigations:
Glycosaminoglycan (GAG) layer defects — exploring targeted GAG replacement therapies beyond PPS and heparin.
Mast cell activation — role in bladder wall inflammation and potential for anti-mast cell biologics.
Nerve growth factor (NGF) — elevated in IC/BPS urine; blocking NGF may reduce pain signaling.
2. Emerging Oral Therapies
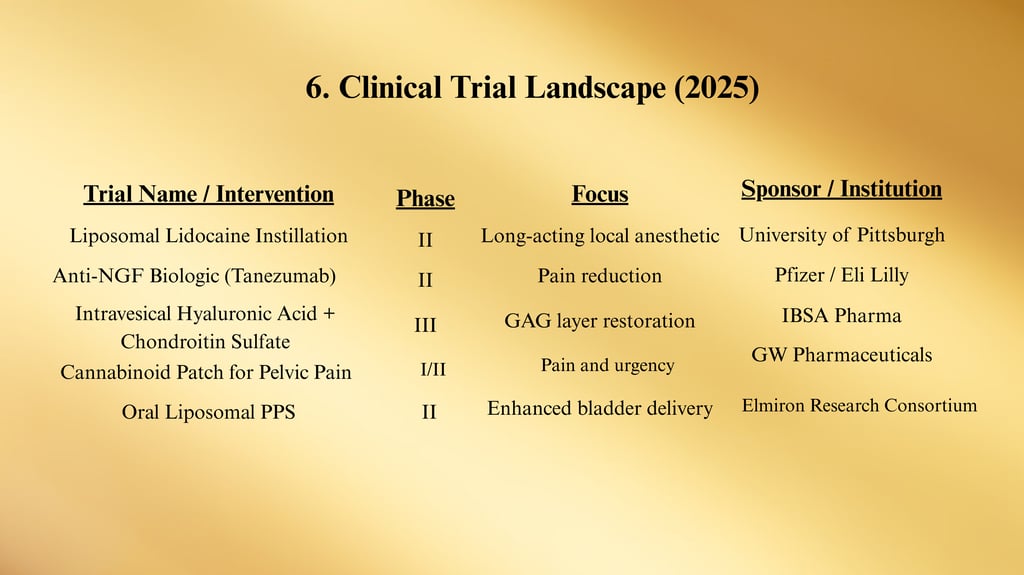
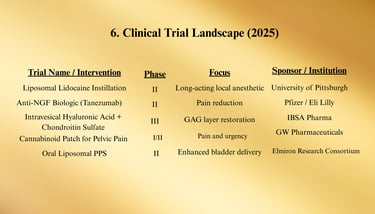
a. Anti-NGF Agents (e.g., Tanezumab)
Mechanism: Monoclonal antibody blocking nerve growth factor activity.
Status: Phase II trials for pelvic pain conditions, including IC/BPS.
b. Cannabinoid-Based Therapies
Target: Endocannabinoid receptors in bladder tissue to reduce inflammation and nociception.
Formulations: Oral capsules, intravesical solutions, and transdermal patches under development.
c. Oral Liposomal PPS
New formulation aimed at increasing bladder uptake and reducing systemic exposure.
3. Next-Generation Intravesical Agents
a. Liposome-Encapsulated Lidocaine
Extended release within bladder for long-lasting pain relief.
b. Chondroitin Sulfate + Hyaluronic Acid Combinations
Double-action GAG therapy being tested for better epithelial repair.
c. Botulinum Toxin Nanocarriers
Non-injection delivery via liposomes to reduce invasiveness.
4. Device-Based Innovations
a. Implantable Drug Delivery Systems
Small, flexible devices placed in the bladder to release medication slowly over weeks (similar to IUD concept).
Example: TARIS Biomedical's lidocaine delivery platform.
b. Advanced Neuromodulation
Fully implantable tibial nerve stimulators for home-based use.
Closed-loop systems that adjust stimulation in real time based on bladder activity.
5. Biomarker Discovery
Efforts to create non-invasive diagnostic tests using urine, blood, or tissue biomarkers could revolutionize early detection and treatment matching.
Potential markers: IL-6, IL-8, antiproliferative factor (APF), NGF.
Goal: Develop point-of-care tests that confirm IC/BPS subtype and guide therapy selection.

7. Regenerative Medicine Approaches
a. Stem Cell Therapy
Mesenchymal stem cells (MSCs) under study for bladder tissue regeneration and immune modulation.
b. Platelet-Rich Plasma (PRP)
Injections into bladder wall to stimulate healing; early pilot studies report symptom improvement.
8. Patient-Centered Research Trends
Telehealth-Based IC Care: Remote pelvic floor therapy, diet counseling, and symptom monitoring via smartphone apps.
Personalized Treatment Algorithms: Using AI to match patients to therapies based on symptom profiles, comorbidities, and genetic factors.
Global IC Registries: Large-scale patient data collection to track long-term outcomes and identify best practices.
9. Challenges and Future Directions
Key Barriers
Heterogeneity of disease presentation.
Lack of a definitive diagnostic test.
Limited funding compared to more common urological conditions.
Optimistic Outlook
Increasing collaboration between urology, pain medicine, immunology, and neuroscience.
Push toward precision medicine — treating IC/BPS not as one disease, but as multiple distinct phenotypes.
Expanded Literature Review (2019–2025)
This review synthesizes the most relevant and impactful research on Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) published between 2019 and early 2025.
The focus is on epidemiology, pathophysiology, diagnostics, therapeutics, and emerging research directions, drawing on global studies to present a comprehensive view of current knowledge.
1. Epidemiology and Disease Burden
Zhang et al. (2020) — Epidemiology of IC/BPS in Asian Populations (Journal of Urology)
Found prevalence rates ranging from 0.26% to 3.4% depending on diagnostic criteria used.
Highlighted underdiagnosis in men due to symptom overlap with chronic prostatitis.
Clemens et al. (2021) — US IC/BPS Population Estimates (Urology)
Based on NHANES data, estimated 3–8 million women and 1–4 million men in the US meet IC/BPS symptom criteria.
Noted significant impact on work productivity and quality of life.
2. Diagnostic Advances
AUA Guidelines Panel (2022) — Updated IC/BPS Diagnostic and Treatment Algorithm
Emphasized phenotyping patients into Hunner lesion vs. non-Hunner lesion subtypes.
Recommended cystoscopy for persistent cases or suspected Hunner lesions.
Jiang et al. (2023) — Urinary Biomarkers for IC/BPS Diagnosis (Frontiers in Urology)
IL-6, IL-8, and antiproliferative factor (APF) demonstrated high sensitivity for bladder-centric disease.
Potential for point-of-care urine tests in development.
3. Pathophysiology Research
Keay et al. (2020) — Antiproliferative Factor (APF) in IC/BPS (American Journal of Physiology – Renal Physiology)
Demonstrated APF-induced inhibition of urothelial cell proliferation and altered tight junction protein expression.
Supports theory of impaired epithelial barrier function as a core mechanism.
Moldwin & Shoskes (2022) — Central Sensitization in IC/BPS (Nature Reviews Urology)
Proposed that a subset of IC/BPS patients exhibit pain hypersensitivity driven by central nervous system changes, explaining poor response to bladder-targeted therapy.
4. Therapeutic Developments
Oral Therapies
Nickel et al. (2020) — Efficacy of Amitriptyline (Journal of Urology)
RCT demonstrated amitriptyline improved global response assessment (GRA) scores at 12 weeks, especially in patients without severe comorbid pain syndromes.
Han et al. (2021) — Long-Term PPS Safety and Retinal Toxicity Risk (British Journal of Ophthalmology)
Confirmed association between PPS exposure and pigmentary maculopathy, prompting updated screening guidelines.
Intravesical Therapies
Seth et al. (2022) — DMSO Combination Cocktails (Urologic Clinics of North America)
Mixed DMSO with heparin, lidocaine, and sodium bicarbonate showed superior flare control compared to monotherapy.
5. Procedural Interventions
Yoshimura et al. (2019) — Botulinum Toxin A for IC/BPS (Pain Medicine)
Multiple-site intradetrusor BoNT-A injection improved urgency, frequency, and pain scores, with benefits lasting up to 9 months.
Ryu et al. (2021) — Hunner Lesion Fulguration Outcomes (International Neurourology Journal)
78% of patients reported significant symptom improvement at 12 months; recurrence rate was ~40%.
6. Experimental and Future Therapies
Peters et al. (2023) — Implantable Intravesical Lidocaine Delivery Device (Journal of Controlled Release)
Early feasibility trial showed sustained release over 14 days with marked reduction in pain and frequency.
Bross et al. (2024) — Mesenchymal Stem Cell Therapy in IC/BPS (Stem Cell Research & Therapy)
Pilot study suggested improvement in bladder capacity and epithelial integrity at 6 months.
ClinicalTrials.gov — As of January 2025, 28 active trials registered for IC/BPS, including anti-NGF biologics, cannabinoid-based therapies, and regenerative medicine approaches.
7. Key Takeaways from 2019–2025 Literature
Phenotype-based treatment is gaining traction — tailoring therapy to Hunner lesion vs. non-Hunner lesion IC/BPS is more effective than “one-size-fits-all” care.
Combination therapy (oral + intravesical + lifestyle modification) remains the most evidence-supported strategy.
Emerging biologics and regenerative approaches offer promise but require larger, multi-center trials before routine use.
Safety monitoring — particularly with PPS and BoNT-A — is now more clearly defined in guidelines.
Diagnostic biomarker research is progressing toward clinically deployable urine tests.
Evidence Summary Table: Botulinum Toxin A in IC/BPS
Abbreviations: BoNT-A = onabotulinumtoxinA; RCT = randomized controlled trial; GRA = Global Response Assessment; VAS = visual analog scale; UUI = urgency urinary incontinence; CIC = clean intermittent catheterization.
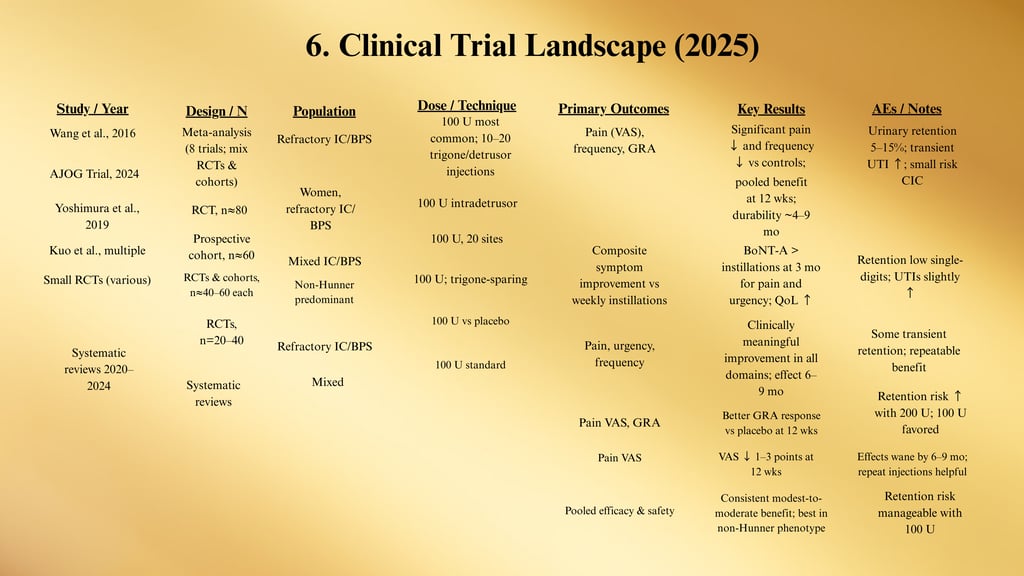

Clinical Takeaways
Who benefits most: Non-Hunner IC/BPS, refractory to oral/instillation therapy, without severe pelvic floor hypertonicity.
Dose that balances efficacy/safety: 100 U onabotulinumtoxinA. (200 U raises retention without clear added benefit.)
Injection map: 10–20 intradetrusor injections (0.5 mL/site), trigone-sparing in most protocols.
Onset & durability: Onset ~1–2 weeks; benefit commonly 4–9 months. Many patients opt for repeat injections 2–3×/year.
Comparative edge: RCT data show superior short-term symptom relief vs. bladder instillations in some cohorts (particularly for pain/urgency at 3 months).
Adverse events:
Urinary retention: ~5–15% (dose- and technique-dependent); counsel on short-term CIC if needed.
UTI: Mild increase for several weeks post-procedure.
Transient dysuria/hematuria common for 24–72 hrs.
When to not use BoNT-A: Baseline high post-void residual, inability/unwillingness to perform CIC, active UTI, pregnancy, neuromuscular junction disorders.
Quick Protocol
Pre-op: UA/culture (treat UTI first); document PVR; consent re: retention/CIC.
Prep: 100 U BoNT-A reconstituted in 10 mL NS (example); cystoscopic injection 20 sites, avoiding trigone; local or short general anesthesia.
Post-op: Consider single-dose antibiotic per local protocol; teach CIC if high risk; follow-up 2–3 weeks to check PVR and symptoms.
Maintenance: Reassess at 3 months; consider repeat at 4–9 months based on response.
rule out look-alikes fast, 2) classify Hunner vs non-Hunner disease, 3) combine first-line measures with targeted medical/procedural options, and 4) measure and adjust every 8–12 weeks.
Clinical Pathways
A. Primary-Care Intake Pathway
(6–8 weeks)
Purpose: Rapid exclusion of infection/malignancy and launch of first-line care before specialty referral.
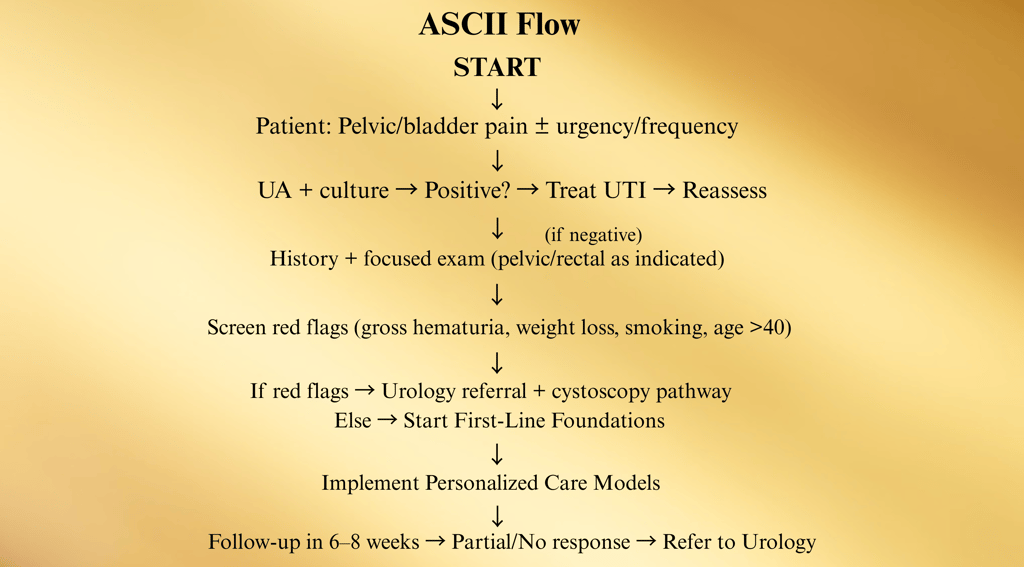

First-Line Foundations to Start in Primary Care
Education (set expectations; chronic condition, stepwise care).
IC elimination diet and hydration optimization.
Bladder training/timed voids.
Stress-reduction plan (CBT, mindfulness, sleep hygiene).
Pelvic floor PT referral (myofascial release/relaxation focus, not Kegels unless indicated).
Symptom diary + trigger log.
Referral triggers from primary care:
Persistent symptoms ≥6–8 weeks.
Hematuria/risk factors for malignancy.
Severe pain, severe QoL impact, or diagnostic uncertainty.
B. Specialty (Urology/Uro-Gyn) Diagnostic Pathway
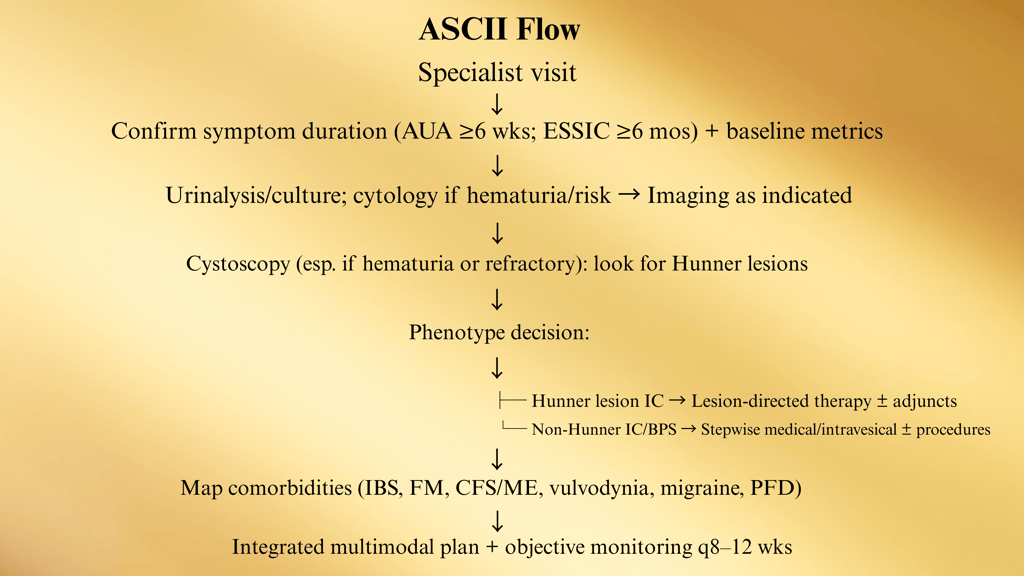
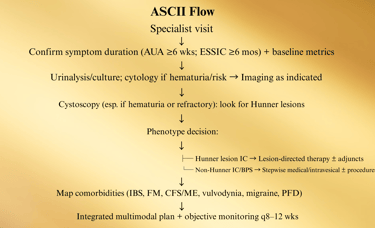
C. Phenotype-Driven Care Maps
1) Hunner Lesion IC (Bladder-centric inflammatory subtype)
Why: This group responds best to lesion therapy—don’t waste months on generic meds only.
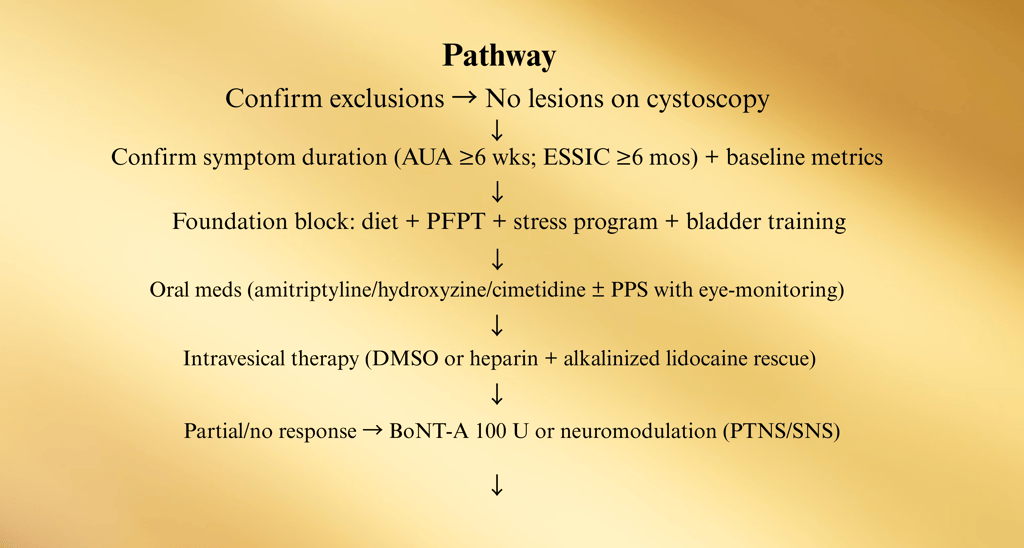
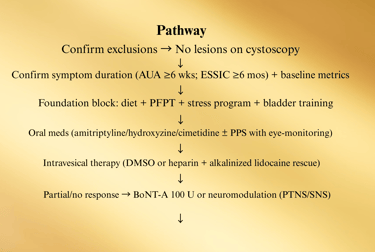
Sev D. Comorbidity-Integrated Pathway (UCPPS overlap)
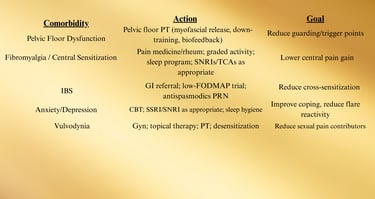
Screen at specialty intake (brief questionnaires acceptable). Route in parallel; don’t “sequence” months apart.
ere refractory, bladder-centric subset → consider hydrodistention

E. Medication & Instillation Ladder (12–24 weeks)
Start foundation (diet, PFPT, stress) + choose ONE oral
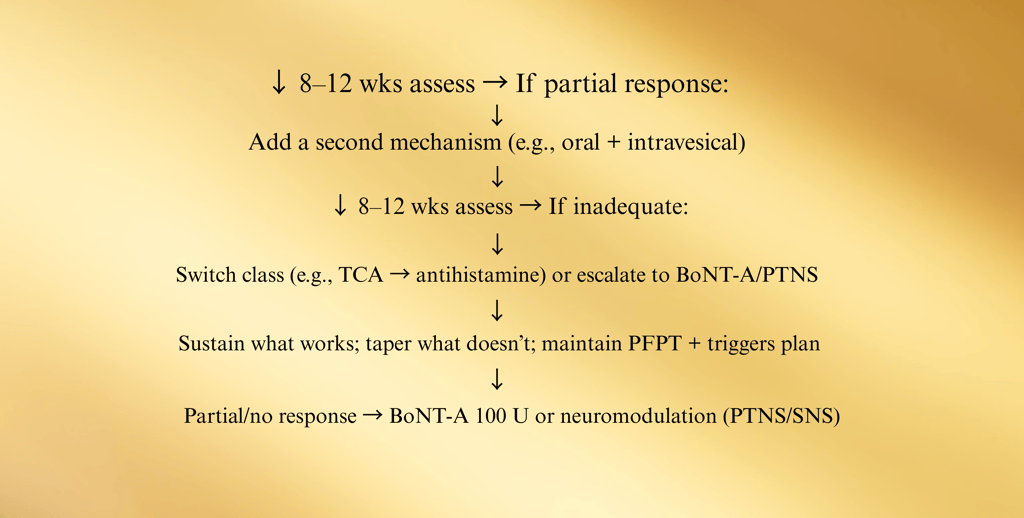
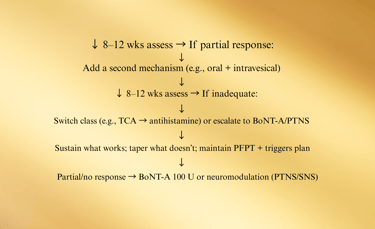
Checkpoints (every 8–12 weeks):
Pain VAS (0–10), voids/24 h, nocturia, urgency episodes, GRA, flares/month, work days lost.
PVR if retention symptoms or after BoNT-A.
PPS users: baseline + annual ophthalmology
F. Procedural Pathway (Refractory cases)
Failure of oral + intravesical + PFPT → Consider:
• Hydrodistention (low pressure, short duration)
• BoNT-A 100 U (trigone-sparing; counsel on CIC risk)
• Neuromodulation (PTNS → SNS if good trial response)
If Hunner lesions present at any time → lesion-directed therapy first
G. Flare Protocol (Patient-issued plan)
Goal: Treat flares fast; avoid ER ping-pong.
Home measures (24–48 h):
Heat/ice as tolerated; pelvic rest; hydration steady (not excessive).
Diet: revert to safest list; avoid acid/spice/caffeine/alcohol.
OTC phenazopyridine short course (warn about urine discoloration).
Prelief® before unavoidable acidic foods.
Clinic measures (as needed):
Rescue instillation: alkalinized lidocaine ± heparin.
Short course bladder-directed analgesia per protocol.
Rule out UTI if new dysuria/fever.
H. Monitoring, Milestones, and Exit Criteria
Visit cadence:
Initiation phase: q8–12 weeks until stable.
Maintenance: q3–6 months; sooner if flare pattern changes.
Milestones (aim within 12–24 weeks):
≥30–50% pain reduction (VAS or PGIC/GRA).
Voids/day reduced by ≥2–3; nocturia down by ≥1.
Flares/month cut by ≥50%.
Return to baseline work/sleep/sexual function goals.
When to step down/de-escalate:
Stable ≥3–6 months: taper least-effective agents; keep foundations + PFPT.
When to step up:
No material progress at 12–16 weeks despite adherence → escalate to next step.
Frequent flares requiring clinic rescue → add preventive intravesical schedule or consider BoNT-A/PTNS.
I. Red Flags (divert off pathway immediately)
Gross hematuria, unexplained weight loss, recurrent UTIs with fever, new neurologic deficits, rapid rising PVR/inability to void, history of pelvic malignancy/radiation with new symptoms.
Pregnant patients with severe pain or bleeding → obstetric co-management.


K. Pocket Algorithm
Suspected IC/BPS
→ UA/culture (treat UTI if +)
→ Screen red flags → cystoscopy if present
→ Start First-Line (diet, PFPT, bladder training, stress plan)
→ 8–12 wks: if partial → add oral (TCA/anti-H1/H2 or PPS) or instillations
→ 8–12 wks: if inadequate → switch/add; consider BoNT-A 100 U or PTNS/SNS
→ Hunner lesions at any point → lesion therapy first
→ Stable ≥3–6 mos → de-escalate what’s not helping; keep foundations
→ Flares → home protocol + rescue instillation as needed
Case Studies
Case 1 — Classic Hunner Lesion IC with Durable Response to Lesion Therapy
Presentation: 54-year-old woman, 2-year history of suprapubic pain (8/10), frequency q45–60 min, nocturia ×4, flares with acidic foods. Prior antibiotics for “recurrent UTI” despite negative cultures.
Workup: UA/culture negative; PVR 30 mL; pelvic exam: pelvic floor tenderness but not hypertonic. Cystoscopy (indicated for hematuria and refractory symptoms): two 1–2 cm inflamed patches with oozing—consistent with Hunner lesions.
Diagnosis/Phenotype: IC/BPS, Hunner-lesion phenotype (bladder-centric).
Treatment: Endoscopic fulguration of lesions + triamcinolone injections; short-duration, low-pressure hydrodistention; adjunct oral amitriptyline 10→25 mg qhs; diet normalization.
Outcome: Pain ↓ to 2/10 by week 3; frequency to every 2–3 h; nocturia ×1. At 18 months, mild recurrence → repeat fulguration with similar benefit.
Lessons Learned: In lesion-positive disease, treat the lesion early—often faster and more durable relief than month-long medication trials
Case 2 — Non-Hunner IC with Pelvic Floor Dysfunction (PFD) Dominance
Presentation: 29-year-old man, 9 months of perineal/bladder pain, post-ejaculatory ache, urgency/frequency, worse under stress.
Workup: UA/culture negative; STI screen negative; DRE: levator ani tenderness; PVR 20 mL. Cystoscopy: no lesions.
Diagnosis/Phenotype: IC/BPS, non-Hunner phenotype with prominent PFD.
Treatment: Pelvic floor physical therapy (myofascial release & down-training), bladder training, stress program; amitriptyline 10→20 mg qhs; rescue alkalinized lidocaine instillation for flares.
Outcome (12 weeks): Pain 7/10 → 3/10; voids 14/day → 8/day; resumed workouts.
Lessons Learned: When PFD drives symptoms, PFPT is first-line therapy; meds work better once the pelvic floor is down-trained.
Case 3 — Refractory Non-Hunner IC Responding to BoNT-A 100 U
Presentation: 41-year-old woman, 3-year non-Hunner IC; failed amitriptyline, hydroxyzine, PPS (partial), and heparin/lidocaine instillations.
Workup: UA/culture negative; cystoscopy: no lesions; PVR baseline 40 mL.
Diagnosis/Phenotype: IC/BPS, non-Hunner refractory.
Treatment: Intradetrusor onabotulinumtoxinA 100 U (20 sites, trigone-sparing) under brief anesthesia; CIC teaching just in case; single-dose antibiotic per protocol.
Outcome: Onset day 10; pain ↓, urgency/frequency improved; PVR rose to 120 mL for 2 weeks → short-term CIC; effect lasted ~7 months; patient opted for repeat at 8 months.
Lessons Learned: 100 U BoNT-A balances efficacy/retention risk; set expectations about possible temporary CIC.
Timeline (months):
0 1 2 3 4 5 6 7 8
|----BoNT-A effect--------------| Repeat
Case 4 — Neuromodulation After Multimodal Failure
Presentation: 46-year-old woman, post-hysterectomy onset; severe urgency, frequency (q30–45 min), sleep disruption; failed PFPT + oral + instillations.
Workup: UA/culture negative; cystoscopy: no lesions; PVR 35 mL.
Diagnosis/Phenotype: IC/BPS, non-Hunner, refractory.
Treatment: Percutaneous tibial nerve stimulation (PTNS) ×12 weekly sessions → partial benefit; proceeded to sacral neuromodulation (SNS) two-stage implant after >50% improvement on trial.
Outcome: Voids down to q2–3 h; nocturia 3→1; pain 6/10→3/10; QoL markedly improved at 12 months.
Lessons Learned: SNS can deliver durable improvements when conservative and pharmacologic options plateau; a trial first minimizes unnecessary implants.
Case 5 — PPS-Associated Maculopathy: Risk Recognition and Transition Plan
Presentation: 67-year-old woman, 12 years on PPS (300 mg/day). New difficulty reading, metamorphopsia.
Workup: Ophthalmology: pigmentary maculopathy on OCT; urinary symptoms stable but not in remission.
Diagnosis/Phenotype: Non-Hunner IC with PPS-associated maculopathy.
Treatment: PPS discontinued; switched to amitriptyline + PRN rescue instillations; ophthalmology follow-up q6–12 months.
Outcome: Vision stabilized; bladder symptoms manageable with combination regimen.
Lessons Learned: Screen eyes at baseline and annually for long-term PPS users; have an exit strategy if toxicity emerges.
Case 6 — IC/BPS with Central Sensitization (Fibromyalgia/IBS Overlap)
Presentation: 39-year-old woman with IC/BPS, fibromyalgia, and IBS; widespread pain; frequent flares under stress.
Workup: UA/culture negative; cystoscopy without lesions; PROMs show high pain catastrophizing score.
Diagnosis/Phenotype: IC/BPS, non-bladder-centric with central sensitization.
Treatment: Interdisciplinary plan—PFPT; low-dose amitriptyline; graded activity; sleep program; CBT; GI: low-FODMAP trial.
Outcome (16 weeks): Pain VAS 8→4; flares/month 5→2; work attendance improved.
Lessons Learned: In overlap syndromes, treat the system, not just the bladder; outcomes hinge on addressing central drivers.
Case 7 — Hydrodistention as Bridge + Lesion Discovery
Presentation: 33-year-old woman, severe frequency and bladder pain; second-line meds and instillations helped minimally.
Workup: UA/culture negative; planned low-pressure hydrodistention; under anesthesia, small focal bleeding area identified consistent with early Hunner lesion.
Diagnosis/Phenotype: Transition from suspected non-Hunner to Hunner-positive disease.
Treatment: Gentle hydrodistention performed; lesion treated with fulguration in same session; restart foundations + rescue instillations as needed.
Outcome: Immediate post-op flare (1 week) then steady improvement; at 6 months, pain 7→2, frequency halved.
Lessons Learned: Hydrodistention can unmask small lesions and offer short-term relief; combine with lesion therapy when present
Case 8 — Pregnancy with IC/BPS: Conservative, Safe Path
Presentation: 30-year-old woman, known non-Hunner IC; becomes pregnant; worsening nocturia and urgency.
Workup: Recurrent UA/cultures (pregnancy increases UTI risk); avoid unnecessary invasive testing.
Diagnosis/Phenotype: IC/BPS in pregnancy, non-Hunner.
Treatment (safety-first): Emphasize dietary triggers, hydration titration, PFPT focused on relaxation, behavioral sleep strategies, limited acetaminophen PRN, avoid higher-risk meds/procedures; coordinate OB + urology.
Outcome: Symptoms fluctuated but remained manageable; healthy delivery; postpartum, re-introduced prior regimen.
Lessons Learned: In pregnancy, lean on foundations and PFPT, rigorous UTI screening, and careful shared decision-making.
Phenotype matters: Lesion-positive disease responds fastest to lesion-directed therapy.
PFPT is pivotal when pelvic floor hypertonicity is present—often the unlock for meds to work.
BoNT-A 100 U is a pragmatic sweet spot (set expectations for temporary retention).
Neuromodulation (trial → implant) helps selected refractory patients regain function.
Safety vigilance: PPS users need baseline/annual eye exams and a prepared alternative plan.
Overlap syndromes: Outcomes improve when you co-manage central sensitization, IBS, mood/sleep.
Hydrodistention (low-pressure, short-duration) can both diagnose and bridge.
Pregnancy: conservative care and OB-urology collaboration keep patients safe.
Cross-Case Themes (What these teach your team)
Presentation: 67-year-old woman, 12 years on PPS (300 mg/day). New difficulty reading, metamorphopsia.
Workup: Ophthalmology: pigmentary maculopathy on OCT; urinary symptoms stable but not in remission.
Diagnosis/Phenotype: Non-Hunner IC with PPS-associated maculopathy.
Treatment: PPS discontinued; switched to amitriptyline + PRN rescue instillations; ophthalmology follow-up q6–12 months.
Outcome: Vision stabilized; bladder symptoms manageable with combination regimen.
Lessons Learned: Screen eyes at baseline and annually for long-term PPS users; have an exit strategy if toxicity emerges.
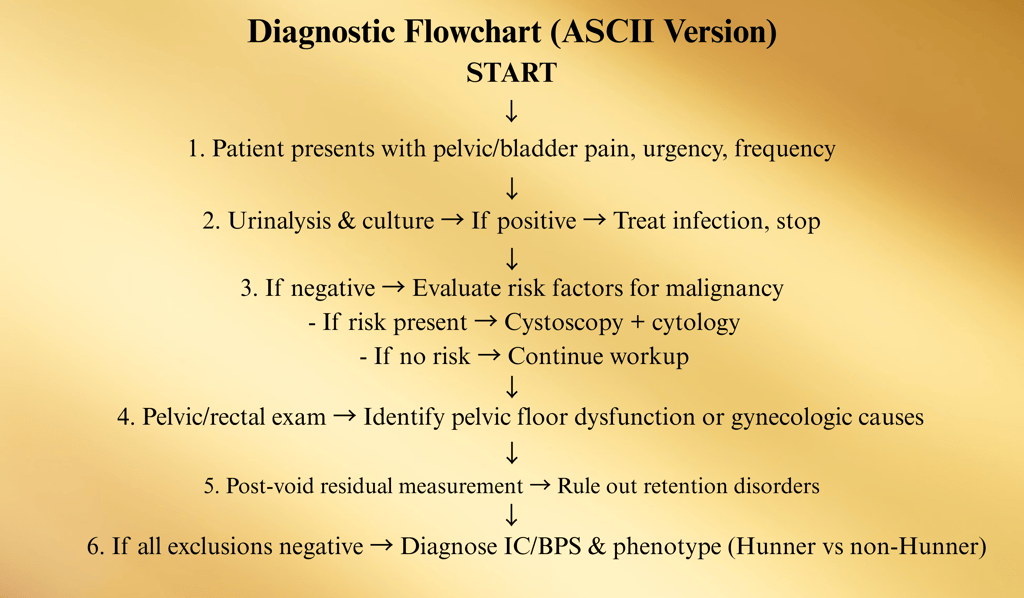
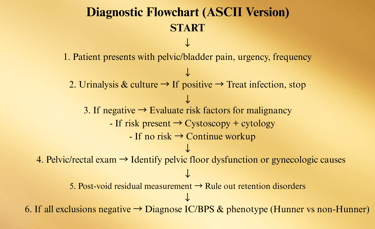
Flowchart: Diagnostic Algorithm
Patient presents with:
• Bladder/pelvic pain, pressure, discomfort
• ± Urinary urgency, frequency, nocturia






Additional Case Studies
Presentation: 37-year-old man, 14-month history of perineal pain, frequency (voiding q1h), and nocturia ×3; previously treated multiple times for “prostatitis” with antibiotics without relief.
Workup: UA/culture negative; STI screen negative; prostate exam normal; cystoscopy: no lesions. PVR: 15 mL.
Diagnosis/Phenotype: Non-Hunner IC/BPS.
Treatment: Education, IC diet, pelvic floor physical therapy (focus on down-training), low-dose amitriptyline, rescue intravesical alkalinized lidocaine for flares.
Outcome: At 12 weeks, pain ↓ from 7/10 to 3/10, nocturia down to 1–2; resumed full work shifts.
Lessons Learned: Men with IC/BPS are often misdiagnosed as prostatitis — early cystoscopy and symptom pattern recognition can save months of ineffective antibiotics.
Case 9 — Male IC/BPS Misdiagnosed as Chronic Prostatitis
Case 10 — High-Pressure Hydrodistention Complication
Presentation: 43-year-old woman, known non-Hunner IC/BPS, underwent hydrodistention at outside hospital (pressures >100 cm H₂O, duration >15 minutes).
Workup: Post-procedure urinary retention (PVR 450 mL), bladder spasms, hematuria. Imaging ruled out perforation.
Treatment: CIC for 2 weeks, anticholinergics for spasms, gradual recovery; transitioned to low-pressure, short-duration hydrodistention protocol at our center with no repeat complications.
Outcome: Improved symptoms after safer re-treatment; resumed normal voiding.
Lessons Learned: High-pressure, prolonged hydrodistention carries increased complication risk — AUA guidelines recommend low pressure (<80 cm H₂O) and short duration (<10 min).
Case 11 — IC/BPS with Autoimmune Overlap
Presentation: 50-year-old woman, IC/BPS symptoms plus dry eyes/mouth; ANA positive; diagnosed with Sjögren’s syndrome.
Workup: Cystoscopy: mild glomerulations, no Hunner lesions; UA negative; Schirmer test abnormal.
Diagnosis/Phenotype: Non-Hunner IC/BPS with autoimmune overlap.
Treatment: Coordinated care with rheumatology; hydroxychloroquine for Sjögren’s; oral cimetidine for bladder symptoms; PFPT; trigger avoidance.
Outcome: Bladder pain decreased by 50%, overall fatigue improved with systemic treatment.
Lessons Learned: Autoimmune disease can exacerbate bladder symptoms — systemic disease control can improve IC/BPS outcomes.
Presentation: 14-year-old girl, 8-month history of urinary urgency, frequency, and pelvic discomfort; significant school absences.
Workup: UA/culture negative; ultrasound normal; cystoscopy under anesthesia revealed no lesions.
Diagnosis/Phenotype: Non-Hunner IC/BPS (pediatric onset).
Treatment: Education for patient/family, dietary trigger management, bladder training, school accommodations, biofeedback-assisted PFPT.
Outcome: Marked symptom improvement; returned to full school attendance.
Lessons Learned: IC/BPS can occur in children/adolescents — early recognition and family involvement in care plan are critical
Presentation: 58-year-old woman, 20-year history of Hunner-lesion IC; multiple lesion fulgurations, BoNT-A injections, neuromodulation — all provided temporary relief.
Workup: Cystoscopy: diffuse Hunner lesions, bladder capacity severely reduced (~100 mL under anesthesia).
Diagnosis/Phenotype: End-stage Hunner-lesion IC/BPS with contracted bladder.
Treatment: After multidisciplinary consult and counseling, underwent ileal conduit urinary diversion.
Outcome: Pain largely resolved post-op; regained mobility and quality of life.
Lessons Learned: Urinary diversion is a last resort but can be life-changing for carefully selected, truly refractory end-stage IC/BPS
Case 12 — Pediatric IC/BPS
Case 13 — Severe Refractory IC/BPS Leading to Urinary Diversion
Literature Spotlight: Microbiome in IC/BPS
Overview
Historically, IC/BPS was framed as a sterile bladder condition.
Recent next-generation sequencing (NGS) and 16S rRNA metagenomics have overturned that view, revealing that both healthy and IC/BPS bladders harbor low-biomass microbial communities — the urobiome.
In IC/BPS, these communities are altered in composition, diversity, and functional potential. The role of these shifts remains under investigation: cause, effect, or bidirectional loop.


Mechanistic Hypotheses
1. Barrier Disruption → Microbial Translocation
o Urothelial tight junction injury allows microbial products (e.g., LPS, peptidoglycan) to trigger local immune activation.
2. Metabolite Signaling
o Certain commensals produce anti-inflammatory short-chain fatty acids (SCFAs); loss of these may perpetuate inflammation.
3. Biofilm Reservoir Hypothesis
o Some IC/BPS patients harbor low-grade biofilms in bladder wall niches → persistent immune activation despite negative standard cultures.
4. Cross-Organ Crosstalk
Gut dysbiosis may influence bladder via systemic immune priming, the microbiome–gut–bladder axis.
Diagnostic & Therapeutic Implications
Biomarker Potential:
Distinct microbial signatures may predict Hunner vs non-Hunner phenotypes or treatment response.Microbiome-Targeted Therapies (Experimental):
Probiotics/prebiotics tailored to bladder/gut species.
Postbiotics (SCFA supplementation).
Targeted antimicrobials against biofilm-forming anaerobes.
Fecal microbiota transplantation (FMT) — only anecdotal evidence in IC/BPS.
Limitations:
Contamination risk in low-biomass samples.
Need for standardization in sample collection (catheterized urine vs suprapubic aspirate).
Inter-study variability in sequencing depth and analytic pipelines.
Research Gaps (as of 2025)
1. Longitudinal Cohorts — Are microbiome changes stable, progressive, or fluctuating with flares?
2. Causality Studies — Can altering the microbiome reverse symptoms?
3. Phenotype Stratification — Microbiome patterns specific to Hunner vs non-Hunner IC.
4. Intervention Trials — Controlled testing of probiotics, antibiotics, FMT.
Multi-Omics Integration — Link microbiome data with metabolomics, transcriptomics, and immune profiling.
Summary Statement
The urobiome is emerging as a key player in IC/BPS pathophysiology.
Evidence points to altered microbial diversity, enriched anaerobic taxa, and functional gene shifts. While causality remains unproven, microbiome profiling may soon help phenotype patients and personalize therapy.
Policy, Coverage, and Advocacy
1. Policy Landscape
Interstitial Cystitis / Bladder Pain Syndrome (IC/BPS) is recognized as a chronic medical condition by most U.S. healthcare systems, yet gaps in policy and coverage persist.
No universally mandated federal coverage: IC/BPS management is largely guided by private insurance, Medicare, and Medicaid policies, which vary by state and plan.
Clinical Guidelines as Policy Drivers: The American Urological Association (AUA) IC/BPS Guideline serves as the reference point for many payers in determining medical necessity for tests and treatments.
CDC & NIH Recognition: IC/BPS is included in the NIH Chronic Pain Research portfolio and is listed under the NIH Rare Diseases Clinical Research Network in certain contexts.
2. Coverage: U.S. Insurance and Payer Practices
A. Diagnostic Coverage
Typically covered: Urinalysis, urine culture, cystoscopy, imaging (if indicated), bladder diaries, and symptom questionnaires.
Prior authorization may be required for cystoscopy, especially with hydrodistention.


C. Cost Barriers
High copays for brand-only medications (e.g., Elmiron® / PPS).
Out-of-pocket costs for compounded instillations in non-hospital settings.
Travel expenses for specialty care in rural/underserved areas
3. Advocacy Strategies
A. Patient-Level Advocacy
Documentation: Keep detailed symptom logs, failed therapy history, and physician letters to support insurance appeals.
Appeal denials: Submit AUA guidelines excerpts and peer-reviewed literature to demonstrate medical necessity.
Financial Assistance Programs: Many drug manufacturers and nonprofit organizations offer co-pay support.
B. Organizational Advocacy
Nonprofit coalitions: Partner with groups such as:
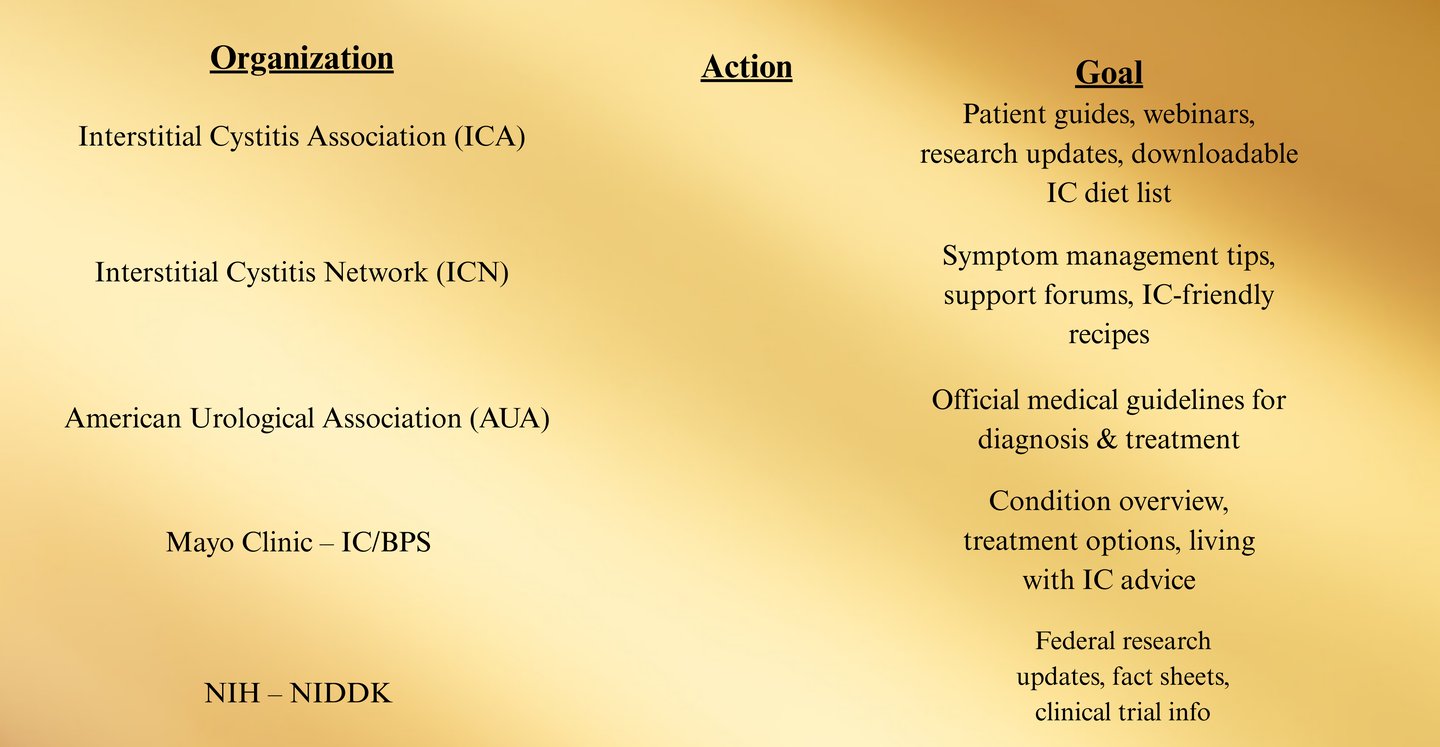
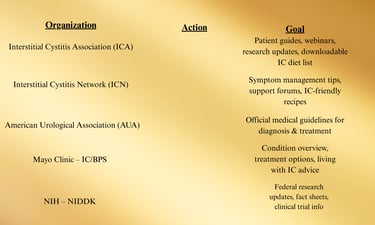
Interstitial Cystitis Association (ICA) — www.ichelp.org
Interstitial Cystitis Network (ICN) — www.ic-network.com
Awareness campaigns: October is IC Awareness Month — opportunities for coordinated media, webinars, and legislative outreach.
C. Legislative & Regulatory Advocacy
Push for Rare Disease Classification: Could expand NIH funding and specialized research grants.
Insurance parity laws: State-level efforts to require equal coverage for chronic pain therapies and emerging interventions.
Medicare coverage expansions: Advocate for coverage of home-administered PFPT tools and TTNS devices.
4. Global Advocacy Perspective
In countries with universal healthcare, IC/BPS treatment access depends on inclusion in national clinical pathways.
The European Association of Urology (EAU) guidelines influence EU payer decisions; national IC patient groups lobby for adoption of coverage for newer therapies such as neuromodulation and BoNT-A.
5. Actionable Recommendations
Educate payers using guideline excerpts to support coverage requests.
Engage policymakers with patient stories + economic impact data (missed workdays, disability claims).
Build coalitions between urology societies, pain advocacy groups, and women’s health organizations.
Leverage telehealth expansion to improve access for rural IC/BPS patients.
Track coverage denials to identify systemic barriers and target advocacy campaigns.
Appendix A: Practical Care Playbook
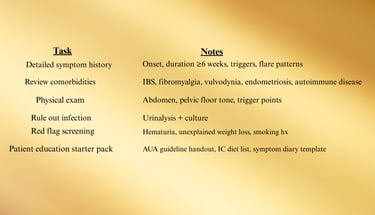
Goal: Confirm diagnosis, phenotype, and patient education.
1. Initial Visit Checklist (Clinician)
Step 1 — Foundations (All patients)
IC education: condition, non-curable but manageable
Trigger identification: diet, stress, activity
Bladder training: timed voids, urge suppression
Pelvic floor physical therapy (PFPT) for hypertonicity
Step 2 — Oral / Intravesical Therapies
Amitriptyline, hydroxyzine, cimetidine, PPS
Intravesical lidocaine, heparin, DMSO (per response)
Step 3 — Minimally Invasive
Botulinum toxin A (100 U recommended for balance)
PTNS / TTNS (nerve stimulation)
Low-pressure, short-duration hydrodistention
Step 4 — Advanced
Sacral neuromodulation (SNS)
Urinary diversion (end-stage, refractory only)
2. Stepwise Care Ladder
3. Patient Self-Management Toolbox
Dietary
Avoid acidic, caffeinated, carbonated, spicy, and artificial sweetener-laden foods during flares.
Use an elimination/reintroduction protocol to identify safe foods.
Flare Survival Kit
Pre-mixed alkalinized lidocaine (Rx) for rescue instillation
Heat or cold packs
OTC urinary analgesics (phenazopyridine — short term only)
Relaxation apps (guided breathing, meditation)
Lifestyle
Stress reduction: yoga, CBT, biofeedback
Sleep hygiene: consistent bedtime, limit evening fluids
Hydration optimization: small, steady sips vs. large volumes
4. Flare Protocols (Patient Version)
5. Clinician Quick-Pick Algorithms
If patient phenotype = Hunner lesion
→ Lesion-directed therapy (fulguration, steroid injection) first, then maintain with foundations.
If phenotype = Non-Hunner + PFD
→ PFPT + bladder retraining before escalating meds.
If phenotype = Central Sensitization overlap
→ Integrate pain psychology, graded activity, sleep restoration early.
Mild Flare
Revert to “safe” diet list
Increase hydration with neutral pH water
Heat pack to suprapubic area
Short course of OTC analgesic if approved
Moderate Flare
Above + use rescue intravesical therapy (if prescribed)
PFPT-guided stretches and pelvic relaxation exercises
Increase mindfulness/relaxation frequency
Severe Flare
Contact urology clinic for same-week evaluation
Consider intravesical therapy or short-term medication adjustment
Rule out UTI with UA/culture
6. Key Forms & Templates (Appendix B Reference)
Symptom Diary (daily)
Food & Flare Tracker
Global Response Assessment (GRA) form
IC Diet Printable Handout
Pelvic Floor Stretching Guide (illustrated)
7. Core Patient Education Links
Interstitial Cystitis Association (ICA) — www.ichelp.org
Interstitial Cystitis Network (ICN) — www.ic-network.com
AUA IC/BPS Guideline — www.auanet.org

Appendix B: Extended Patient Resources
These organizations provide evidence-based information, webinars, printable guides, and clinician directories.
1. Education & Information Hubs
1. 2. Self-Management Tools
Apps & Digital Tools:
FlareTracker (mobile) – Tracks symptoms, diet, and triggers
MyICDiary (web/app) – Printable reports for doctor visits
Calm / Headspace – Mindfulness and stress reduction
Printable Tools:
IC Diet Food List (ICA & ICN versions)
Bladder Diary Templates (AUA)
Pelvic Floor Relaxation Exercise Sheets
3. Peer Support & Community
Online Support Groups:
Facebook Groups: “Living with IC,” “Interstitial Cystitis Support”
Reddit: /r/Interstitialcystitis
HealthUnlocked – IC/BPS Community: Link
Local In-Person Groups:
ICA Local Chapters – listed by state on ICA website
Hospital-affiliated chronic pain support programs
4. Financial Assistance & Insurance Help
Partnership for Prescription Assistance — www.pparx.org
NeedyMeds — www.needymeds.org
PAN Foundation — www.panfoundation.org (for medication copay help)
Manufacturer assistance programs for PPS (Elmiron®), botulinum toxin, and neuromodulation devices.
5. Advocacy & Policy Engagement
IC Awareness Month (October) — Media toolkits via ICA & ICN
NIH Patient Advocacy Groups — Opportunities to join rare disease advocacy events
Chronic Pain Policy Alliance — Lobbying for insurance parity and chronic pain research funding
State-Level Legislative Watch — Contact state urology societies for pending insurance mandates
6. Clinical Trial Participation
Patients may be eligible for research studies testing new medications, neuromodulation devices, or microbiome therapies.
ClinicalTrials.gov Search: IC/BPS Active Trials
Ask your urologist about NIH-funded multi-site IC/BPS studies in your area.
7. Emergency Resources
For severe flares with sudden inability to urinate, high fever, or severe bleeding, seek immediate medical attention.
For emotional distress or crisis, call or text 988 Suicide and Crisis Lifeline (U.S.).
8. Recommended Reading
NIDDK. Interstitial Cystitis (Bladder Pain Syndrome) – Overview. https://www.niddk.nih.gov/health-information/urologic-diseases/interstitial-cystitis-bladder-pain-syndrom
American Urological Association. Diagnosis and Treatment of IC/BPS (2022). https://www.auanet.org/guidelines-and-quality/guidelines/diagnosis-and-treatment-interstitial-of-cystitis/bladder-pain-syndrome-%282022%29
EAU Guidelines on Chronic Pelvic Pain (2025). https://uroweb.org/guidelines/chronic-pelvic-pain
ICS. IC/Hunner lesion terminology. https://www.ics.org/committees/standardisation/terminologydiscussions/icbps
Maeda D, et al. Hunner-type IC. https://pmc.ncbi.nlm.nih.gov/articles/PMC4654580/
Anger JT, et al. National prevalence of IC/BPS (2022). https://pmc.ncbi.nlm.nih.gov/articles/PMC9448885/
Berry SH, et al. Prevalence of BPS symptoms. https://pmc.ncbi.nlm.nih.gov/articles/PMC3513327/
NIDDK. Symptoms & Causes. https://www.niddk.nih.gov/health-information/urologic-diseases/interstitial-cystitis-bladder-pain-syndrome/symptoms-causes
Keay SK, et al. Antiproliferative factor. https://www.pnas.org/doi/10.1073/pnas.0404509101
Kim J, et al. APF signaling & HB-EGF antagonism. https://pmc.ncbi.nlm.nih.gov/articles/PMC4000709/
Kim J, et al. APF signaling review. https://pmc.ncbi.nlm.nih.gov/articles/PMC3256302/
Mukhopadhyay C, 2022. PPS maculopathy review. https://pubmed.ncbi.nlm.nih.gov/35533330/
EyeWiki: PPS maculopathy. https://eyewiki.org/Pentosan_Polysulfate_Maculopathy
Wang J, et al. BoNT-A meta-analysis. https://pmc.ncbi.nlm.nih.gov/articles/PMC5032852/
AJOG 2024: OnabotulinumtoxinA vs instillations. https://pubmed.ncbi.nlm.nih.gov/38768800/
ClinicalTrials.gov NCT04401176. https://clinicaltrials.gov/study/NCT04401176
StatPearls: Sacral Neuromodulation. https://www.ncbi.nlm.nih.gov/books/NBK567751/
Kendall HJ, 2024. Neuromodulation position. https://journals.lww.com/co-urology/fulltext/2024/03000/current_position_of_neuromodulation_for_bladder.6.aspx
Tung A, et al. Direct costs of IC/BPS. https://pubmed.ncbi.nlm.nih.gov/28345436/
Laden BF, et al. Comorbidities in UCPPS. https://pmc.ncbi.nlm.nih.gov/articles/PMC8536792/
Interstitial Cystitis Association. https://www.ichelp.org/
IC Network – 2025 IC Food List. https://www.icnetwork.org/interstitial-cystitis-diet/the-ic-food-lists/
IC Network – Flare guide. https://www.ic-network.com/downloads/icnflareguide.pdf
MedlinePlus – IC resources. https://medlineplus.gov/ency/article/005108.htm
Keay S, et al. HB-EGF/EGF shifts. https://www.goldjournal.net/article/S0090-4295(01)01028-7/fulltext
Fu C, et al. Urinary microbiota. https://bjui-journals.onlinelibrary.wiley.com/doi/10.1111/bju.16439
Jiang P, et al. Gut microbiota MR study. https://www.nature.com/articles/s41598-024-69543-9
Nandwana D, et al. Microbiome review in IC/BPS. https://www.sciencedirect.com/science/article/pii/S2405456925000082
29. RAND Prevalence Studies (Osborne, IC Network, 2023)
Estimated 3.2–7.9 million U.S. women (2.7%–6.5%) and 1–4 million men experience IC/BPS symptoms.
Available at: IC Network prevalence article PMC+9UroToday+9ScienceDirect+9IC Network+2PMC+2
30. Cacciatore et al., 2024 – Comprehensive Review of BPS Treatment Strategies
A broad review of management approaches and therapeutic options for bladder pain syndrome.
DOI: https://doi.org/10.2147/RRU.S387749 Dove Medical Press
31. Frontiers in Pain Research, 2023 – Quality-of-Life Impact
Prospective analysis of health-related quality of life (HRQoL) in IC/BPS compared with other pelvic pain conditions.
SELF+15Frontiers+15ScienceDirect+15
32. MDPI Review, 2023 – Pathomechanism & Current Treatments
Covers inflammation, urothelial biomarkers, BoNT-A, PRP, DMSO, and LESWs in IC/BPS.
PMC
33. StatPearls (NCBI Book), Year Unknown – Clinical Overview
Defines IC/BPS as chronic inflammation under 6+ weeks, explains diagnostic challenges.
Frontiers+15NCBI+15Frontiers+15
34. Medscape (eMedicine), 2024 – Practice Essentials & Pathophysiology
Covers urinary frequency, urgency, pelvic pain, pelvic floor dysfunction, neural hypersensitivity.
SELF+3Medscape+3PubMed+3
35. Frontiers in Physiology, 2023 – Animal Models & Phenotypes
Discusses heterogeneity and prevalence of Hunner lesions (5%–57%) and phenotyping.
SpringerLink+3Frontiers+3AUA Journals+3
36. NCBI Workshop Summary – Translational IC/BPS Research
Overview of current clinical status and future basic/translational research directions.
ScienceDirect
37. Springer 2025 – Matching Therapies to Phenotypes in IC/BPS
Highlights pelvic floor dysfunction, overlapping phenotypes, and a shift to multimodal, personalized treatments.
SpringerLink+1
38. BMC Urology, 2024 – Systematic Review & Meta-Analysis of Treatments
Suggests that combinations of therapies are more effective than single approaches.
BioMed Central
39. AUA Guideline, 2022 – Role of Cystoscopy & Hydrodistension
Recommends cystoscopy for suspected Hunner lesions and guidance on cystoscopy protocols.
International Neurourology Journal+5AUA Journals+5PMC+5
40. Kendall HJ, 2024 – Neuromodulation Advances
Reports promising evidence for sacral, tibial, and pudendal neuromodulation, especially PTNS/TTNS.
Lippincott Journals+2PubMed+2
41. Abdalla et al., 2023 – 11-Year Experience with PTNS
Clinical outcomes from using PTNS in IC/BPS patients.
ScienceDirect+15ScienceDirect+15International Neurourology Journal+15
42. IC Network Patient Report – PTNS Study Outcome
Notes lack of significant improvement in a small PTNS trial (85% reported no effect).
IC Help
43. AUA Note on PTNS Study (20 Women)
Prospective study where 75% reported no improvement after 12 weeks of PTNS.
American University Association
44. Broader Pathophysiology Review (Frontiers 2024)
Positions IC as a chronic primary pain disorder of biopsychosocial nature, urging reframing of treatment.
Frontiers




Disclaimer: The Interstitial Cystitis (IC) references and external links provided are for educational purposes only and do not constitute medical advice. We do not control or endorse the content of third-party sites. Always consult a qualified healthcare provider regarding any medical concerns. Use of linked resources is at your own risk.
Support
Raising awareness and supporting IC patients together.
Advocacy
Community
(832) 580-9972


© 2024. All rights reserved.
